研究室紹介Laboratories
- Back
- Top > 研究室紹介 > 神経疾患病態統御部門 > 神経情報薬理学(薬理学)
神経疾患病態統御部門神経情報薬理学(薬理学)
研究室概要
私達の研究室では、脳の高次機能の基本機能単位であるシナプス機能を制御する分子機構、および脳病態におけるその破綻機構の解明を目指しています。具体的には、記憶や学習の分子基盤をなすと考えられているAMPA型グルタミン酸受容体(AMPA受容体)を介したシナプス伝達の制御機構に着目しています。生化学、分子・細胞生物学、組織学、電気生理学、マウス遺伝学等を駆使して研究を行っています。
研究プロジェクト
私達はこれまでに特異性と定量性を重視した生化学的手法に基づいて、AMPA受容体制御分子として、 (1) パルミトイル化脂質修飾制御酵素と(2) てんかん関連リガンド・受容体LGI1・ADAM22を独自に同定してきました。
(1) 動的なパルミトイル化脂質修飾に関する研究
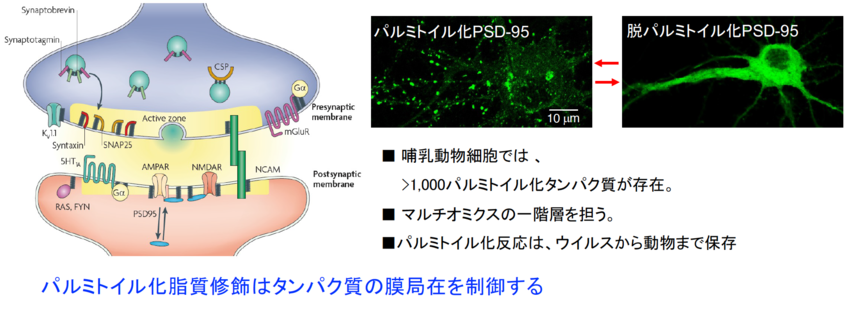
パルミトイル化および脱パルミトイル化酵素ファミリーは、シナプス機能に留まらず、幅広い生命現象に関わっており、国内外の多くの研究者と共同研究を展開しております。パルミトイル化は脂質修飾の中で唯一“可逆性”を有し、様々な機能タンパク質の細胞内局在を動的に制御するユニークな反応系です。近年はオミクス解析(パルミトーム解析)も可能となり、病態モデルにおけるパルミトイル化タンパク質の変容も検討しています。
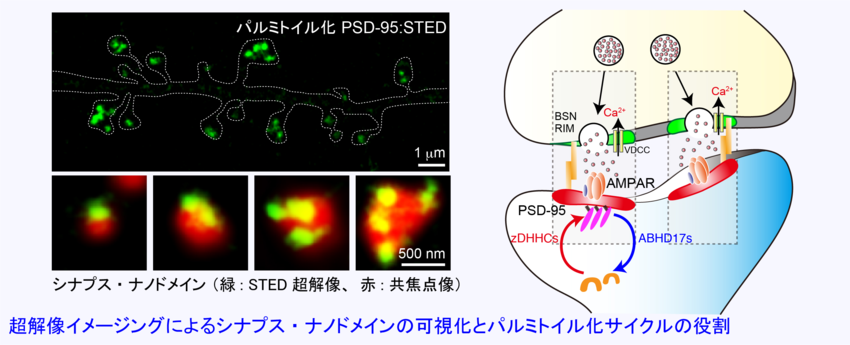
シナプス研究においては、超解像顕微鏡イメージングと独自のパルミトイル化タンパク質の可視化プローブを組み合わせて、シナプス内部にナノドメイン構造を発見しました。興味深いことにシナプス・ナノドメインは、シナプス前部と後部の両方に存在し、両者はナノメートル精度で対面配置されていることが分かってきました(ナノカラム説)。現在は、(i)シナプス・ナノドメインのサイズや個数がどのように制御されているのかについて、および(ii)シナプス・ナノカラムの分子実体とその制御機構について、分子から個体レベルまでをシームレスに跨ぐ研究を進めています。
(2) てんかん関連リガンド・受容体LGI1-ADAM22複合体に関する研究
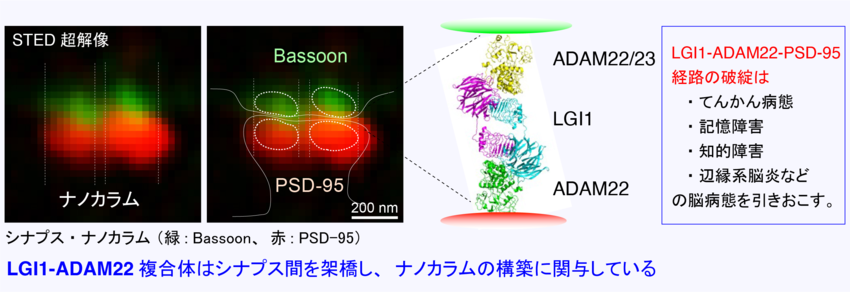
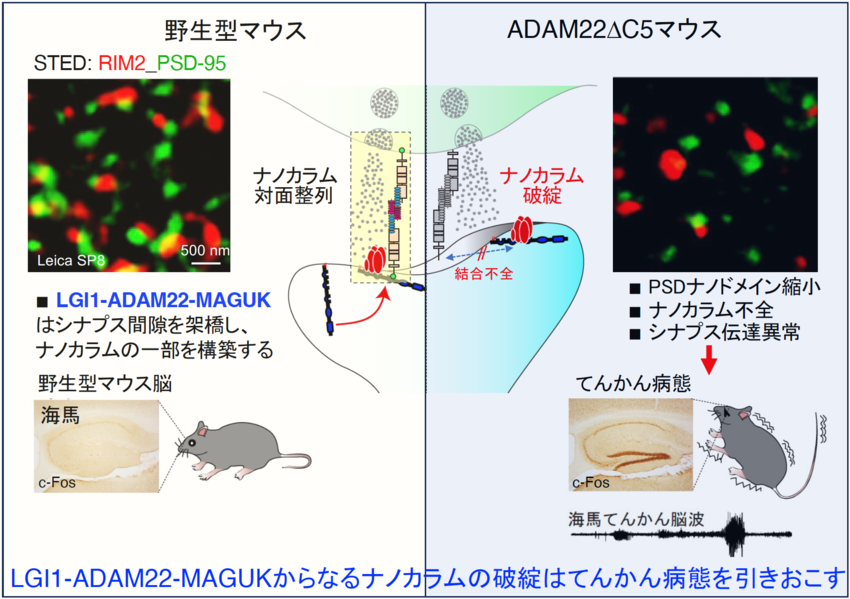
LGI1は神経細胞から分泌されるタンパク質で、多くのバリアント(変異)が側頭葉てんかん患者で報告されています。また、LGI1に対する自己抗体が記憶障害、けいれんを主徴とする自己免疫性辺縁系脳炎で報告され、注目を集めています。私達は、LGI1がADAM22のリガンドとして機能し、興奮性シナプスにおいてシナプス間隙を跨いで高次複合体を形成し、AMPA受容体や電位依存性カリウムチャネル機能を制御することを報告してきました。
一方、私達はLGI1バリアントやLGI1自己抗体、ADAM22バリアントの病態解析を進め、このLGI1-ADAM22間の結合が破綻すると、てんかんや記憶障害、知的障害などの脳病態が生じることを見出しました。さらに、ごく最近になってLGI1やADAM22以外のファミリー分子の病的バリアントも複数見つかってきており、ファミリー全体を俯瞰した研究にも着手しております。
最終的には、脳疾患において、パルミトイル化酵素群やLGI1-ADAM22経路を修飾する薬物の開発を目指したいと考えています。
教員
| 構成員名 | 役職 | 所属 |
|---|---|---|
| 深田 正紀 / FUKATA Masaki | 教授 | 神経情報薬理学 |
| 深田 優子 / FUKATA Yuko | 准教授 | 分子細胞薬理学 |
| 横井 紀彦 / YOKOI Norihiko | 講師 | 神経情報薬理学 |
| 宮﨑 裕理 / MIYAZAKI Yuri | 助教 | 神経情報薬理学 |
| 黒田 啓介 / KURODA Keisuke | 特任准教授 | 卓越大学院推進室・神経情報薬理学 |
研究実績
- 2025年
- Upadhya M, Stumpf A, O’Brien-Cairney J, Cordero-Gómez C, Döring J, Hoffmann J, Mueller S, Fukata Y, van Hoof S, Dhangar D, Atwal A, Rosch R, Woodhall G, Boehm-Sturm P, Fukata M, Kreye J, Schmitz D, Wright SK, Kornau HC, Prüss H. Patient-derived monoclonal LGI1 autoantibodies elicit seizures, behavioral changes and brain MRI abnormalities in rodent models. Brain Behav Immun 126:342-355(2025). doi:https://doi.org/10.1016/j.bbi.2025.02.019 PMID: 39984135
- Yamaguchi T, Okatsu K, Kubota M, Mitsumori A, Yamagata A, Fukata Y, Fukata M, Shibata M, Fukai S. Structural insights into heterohexameric assembly of epilepsy-related ligand–receptor complex LGI1–ADAM22. eLife (2025) reviewed preprint. doi: https://doi.org/10.7554/eLife.105918.1
- Sato R, Adachi R, Yokoi N, Tsujimura K, Egawa R, Hara Y, Fukata Y, Fukata M, Ogi T, Sone M, Kuba H. Loss of neuronal activity facilitates surface accumulation of p75NTR and cell death in avian cochlear nucleus. Neurosci Res (2025). doi: 10.1016/j.neures.2025.01.004
- Hirano Y, Miyazaki Y, Ishikawa D, Inahashi H, Al-Hassnan ZN, Zifarelli G, Bauer P, Alvi JR, Sultan T, Thompson ML, Sezer A, Konuşkan B, Hajir RS, El-Hattab AW, Efthymiou S, Ishida A, Yokoi N, Kornau HC, Schmitz D, Pruss H, Houlden H, Ikegaya Y, Fukata Y*, Fukata M*, Maroofian R*. Biallelic LGI1 and ADAM23 variants cause hippocampal epileptic encephalopathy via the LGI1–ADAM22/23 pathway. Brain (2025). doi: https://doi.org/10.1093/brain/awaf202
- 2024年
- Miyazaki Y, Otsuka O, Yamagata Y, Endo T, Sanbo M, Sano H, Kobayashi K, Inahashi H, Kornau H-C, Schmitz D, Prüss H, Meijer D, Hirabayashi M, Fukata Y*, Fukata M* (*, corresponding authors). Oligodendrocyte-derived LGI3 and its receptor ADAM23 organize juxtaparanodal Kv1 channel clustering for short-term synaptic plasticity. Cell Rep 43:113634 (2024). doi.org/10.1016/j.celrep.2023.113634. PubMed 38194969
- Fukata Y, Fukata M, MacGillavry HD, Nair D, Hosy E. Celebrating the birthday of AMPA receptor nanodomains: Illuminating the nanoscale organization of excitatory synapses with 10 nanocandles. J Neurosci 44:e2104232024 (2024). doi: 10.1523/JNEUROSCI.2104-23.2024
- 2023年
- Zhang X, Kira JI, Ogata H, Imamura T, Mitsuishi M, Fujii T, Kobayashi M, Kitagawa K, Namihira Y, Ohya Y, Maimaitijiang G, Yamasaki R, Fukata Y, Fukata M, Isobe N, Nakamura Y. Anti-LGI4 Antibody Is a Novel Juxtaparanodal Autoantibody for Chronic Inflammatory Demyelinating Polyneuropathy. Neurol Neuroimmunol Neuroinflamm. 10:e200081 (2023). doi: 10.1212/NXI.0000000000200081. PubMed 36631269
- Nosková R*, Fukata Y*, Stránecký V, Šaligová J, Bodnárová O, Giertlová M, Fukata M**, Kmoch S** (*, equally contributed; **, corresponding authors). ADAM22 ethnic-specific variant reducing binding of membrane-associated guanylate kinases causes focal epilepsy and behavioural disorder. Brain Commun (2023). doi.org/10.1093/braincomms/fcad295. PubMed 37953841
- Chen L, Fukata Y, Murata K. In situ cryo-electron tomography: a new method to elucidate cytoplasmic zoning at the molecular level. J Biochem. 2023 Dec 15:mvad102. doi: 10.1093/jb/mvad102. PubMed 38102736
- 2022年
- Niki Y, Adachi N, Fukata M, Fukata Y, Oku S, Makino-Okamura C, Takeuchi S, Wakamatsu K, Ito S, Declercq L, Yarosh D B, Mammone T, Nishigori C, Saito N, Ueyama T. S-palmitoylation of tyrosinase at cysteine500 regulates melanogenesis. J Invest Dermatol (2022). doi: 10.1016/j.jid.2022.08.040. PubMed 36063887
- van der Knoop MM*, Maroofian R*, Fukata Y*, van Ierland Y, Karimiani EG, Lehesjoki AE, Muona M, Paetau A, Miyazaki Y, Hirano Y, Selim L, de França M, Fock RA, Beetz C, Ruivenkamp CAL, Eaton AJ, Morneau-Jacob FD, Sagi-Dain L, Shemer-Meiri L, Peleg A, Haddad-Halloun J, Kamphuis DJ, Peeters-Scholte CMPCD, Hiz Kurul S, Horvath R, Lochmüller H, Murphy D, Waldmüller S, Spranger S, Overberg D, Muir AM, Rad A, Vona B, Abdulwahad F, Maddirevula S, Povolotskaya IS, Voinova VY, Gowda VK, Srinivasan VM, Alkuraya FS, Mefford HC, Alfadhel M, Haack TB, Striano P, Severino M, Fukata M**, Hilhorst-Hofstee Y**, Houlden H**. (*, equally contributed; **, corresponding authors). Biallelic ADAM22 pathogenic variants cause progressive encephalopathy and infantile-onset refractory epilepsy. Brain (2022). doi: 10.1093/brain/awac116. PubMed 35373813
- Kawai T, Narita H, Konno K, Akter S, Andriani RT, Iwasaki H, Nishikawa S, Yokoi N, Fukata Y, Fukata M, Wiriyasermkul P, Kongpracha P, Nagamori S, Takao K, Miyakawa T, Abe M, Sakimura K, Watanabe M, Nakagawa A, Okamura Y. Insight into the function of a unique voltage-sensor protein (TMEM266) and its short form in mouse cerebellum Biochem J. (2022). doi: 10.1042/BCJ20220033. PubMed 35574701
- Wirth A, Labus J, Galil DA, Schill Y, Schmidt S,Bunke T, Gorinski N, Yokoi N, Fukata M. Ponimaskin E Palmitoylation of the small GTPase Cdc42 by DHHC5 modulates spine formation and gene transcription. J Biol Chem. (2022). doi: 10.1016/j.jbc.2022.102048. PubMed 35597282
- 2021年
- Fukata Y, Chen X, Chiken S, Hirano Y, Yamagata A, Inahashi H, Sanbo M, Sano H, Goto T, Hirabayashi M, Kornau HC, Prüss H, Nambu A, Fukai S, Nicoll RA, Fukata M. LGI1-ADAM22-MAGUK configures trans-synaptic nanoalignment for synaptic transmission and epilepsy prevention. Proc Natl Acad Sci USA. 118:e2022580118 (2021). doi.org/10.1073/pnas.2022580118. PubMed 33397806
- Yokoi N, Fukata Y*, Okatsu K, Yamagata A,Liu Y, Sanbo M, Miyazaki Y, Goto T, Abe M, Kassai H, Sakimura K, Meijer D, Hirabayashi M, Fukai S, Fukata M*. 14-3-3 proteins stabilize LGI1-ADAM22 levels to regulate seizure thresholds in mice. Cell Rep. 37:110107 (2021). doi.org/10.1016/j.celrep.2021.110107. PubMed 34910912
- Nakamoto C, Goto Y, Tomizawa Y, Fukata Y, Fukata M, Harpsøe K, Gloriam DE, Aoki K, Takeuchi T. A novel red fluorescence dopamine biosensor selectively detects dopamine in the presence of norepinephrine in vitro. Mol Brain. 14:173 (2021). doi.org/10.1186/s13041-021-00882-8. PubMed 34872607
- Kreye J, Wright SK, van Casteren A, Stöffler L, Machule ML, Reincke SM, Nikolaus M, van Hoof S, Sanchez-Sendin E, Homeyer MA, Gómez CC, Kornau HC, Schmitz D, Kaindl AM, Boehm-Sturm P, Mueller S, Wilson MA, Upadhya MA, Dhangar DR, Greenhill S, Woodhall G, Turko P, Vida I, Garner CC, Wickel J, Geis C, Fukata Y, Fukata M, Prüss H. Encephalitis patient-derived monoclonal GABAA receptor antibodies cause epileptic seizures. J Exp Med. 218:e20210012 (2021). doi.org/10.1084/jem.20210012. PubMed 34546336
- Chen X, Fukata Y, Fukata M, Nicoll R. MAGUKs are essential, but redundant, in long-term potentiation. Proc Natl Acad Sci USA. 118:e2107585118 (2021). doi: 10.1073/pnas.2107585118. PubMed 34244435
- Fukata Y, Hirano Y, Miyazaki Y, Yokoi N, Fukata M. Trans-synaptic LGI1-ADAM22-MAGUK in AMPA and NMDA receptor regulation. Neuropharmacology (2021). doi: 10.1016/j.neuropharm.2021.108628. PubMed 34089731
- Yoshida T, Yamagata A, Imai A, Kim J, Izumi H, Nakashima S, Shiroshima T, Maeda A, Iwasawa-Okamoto S, Azechi K, Osaka F, Saitoh T, Maenaka K, Shimada T, Fukata Y, Fukata M, Matsumoto J, Nishijo H, Takao K, Tanaka S, Okabe S, Tabuchi K, Uemura T, Mishina M, Mori H, Fukai S. Canonical versus non-canonical transsynaptic signaling of neuroligin 3 tunes development of sociality in mice. Nat Commun. 2021 12:1848. doi: 10.1038/s41467-021-22059-6. PubMed 33758193
- 2020年
- Watanabe H, Sano H, Chiken S, Kobayashi K, Fukata Y, Fukata M, Mushiake H, Nambu A. Forelimb movements evoked by optogenetic stimulation of the macaque motor cortex. Nat Commun. 11:3253 (2020). doi: 10.1038/s41467-020-16883-5. PubMed 32591505
- Oda Y, Sugawara T, Fukata Y, Izumi Y, Otani Y, Higashi T, Fukata M, Furuse M. The extracellular domain of angulin-1 and palmitoylation of its cytoplasmic region are required for angulin-1 assembly at tricellular contacts. J Biol Chem. 295:4289-4302 (2020). doi:10.1074/jbc.RA119.010491. PubMed 32079676
- Kornau HC, Kreye J, Stumpf A, Fukata Y, Parthier D, Sammons RP, Imbrosci B, Kurpjuweit S, Kowski AB, Fukata M, Prüss H, Schmitz D. Human CSF monoclonal LGI1 autoantibodies increase neuronal excitability. Ann Neurol. 87(3):405-418 (2020). doi: 10.1002/ana.25666. PubMed 31900946
- 2019年
- Sada R, Kimura H, Fukata Y, Fukata M, Yamamoto H, Kikuchi A. Dynamic palmitoylation determines microdomain localization of two DKK1 receptors, CKAP4 and LRP6, and regulates DKK1 signaling. Sci Signaling. 12:eaat9519 (2019). doi: 10.1126/scisignal.aat9519. PubMed 31744930
- Cao Y, Qiu T, Kathayat R, Azizi SA, Fukata Y, Fukata M, Rice P, Dickinson BC. ABHD10 is an S-depalmitoylase affecting redox homeostasis through peroxiredoxin-5. Nat Chem Biol. 15:1232-1240 (2019). doi: 10.1038/s41589-019-0399-y. PubMed 31740833
- Boncompain G, Herit F, Tessier S, Lescure A, Del Nery E, Gestraud P, Staropoli I, Fukata Y, Fukata M, Brelot A, Niedergang F, Perez F. Targeting CCR5 trafficking to inhibit HIV-1 infection. Science Advances. 5:eaax0821 (2019). doi: 10.1126/sciadv.aax0821. PubMed 31663020
- Kanadome T, Yokoi N, Fukata Y, Fukata M. Systematic Screening of Depalmitoylating Enzymes and Evaluation of Their Activities by the Acyl-PEGyl Exchange Gel-Shift (APEGS) Assay Methods. Mol Biol. 2009:83-98 (2019). doi: 10.1007/978-1-4939-9532-5_7. PubMed 31152397
- Hasegawa D, Ohnishi Y, Koyama E, Matsunaga S, Ohtani S, Nakanishi A, Shiga T, Chambers JK, Uchida K, Yokoi N, Fukata Y, Fukata M. Deleted in colorectal cancer (netrin-1 receptor) antibodies and limbic encephalitis in a cat with hippocampal necrosis. J Vet Intern Med. 33:1440-1445 (2019). doi: 10.1111/jvim.15492. PubMed 30942925
- 2018年
- Yamagata A*, Miyazaki Y*, Yokoi N, Shigematsu H, Sato Y, Goto-Ito S, Maeda A, Goto T, Sanbo M, Hirabayashi M, Shirouzu M, Fukata Y, Fukata M**, Fukai S**. (*, equally contributed; **, corresponding authors). Structural basis of epilepsy-related ligand-receptor complex LGI1-ADAM22. Nat Commun. 9:1546 (2018). doi: 10.1038/s41467-018-03947-w. PubMed 29670100
- Yoshikura N, Kimura A, Fukata M, Fukata Y, Yokoi N, Harada N, Hayashi Y, Inuzuka T, Shimohata T. Long-term clinical follow-up of a patient with non-paraneoplastic cerebellar ataxia associated with anti-mGluR1 autoantibodies. J Neuroimmunol. 319:63-67 (2018). doi: 10.1016/j.jneuroim.2018.04.001. PubMed 29685291
- Fukata M, Yokoi N, Fukata Y. Neurobiology of autoimmune encephalitis. Curr Opin Neurobiol. 48:1-8 (2018). doi: 10.1016/j.conb.2017.07.012. PubMed 28829986
- 2017年
- Fukata Y, Fukata M. Epilepsy and synaptic proteins. Curr Opin Neurobiol, 45:1-8 (2017). doi: 10.1016/j.conb.2017.02.001. PubMed 28219682
- Chen IS, Tateyama M, Fukata Y, Uesugi M, Kubo Y. Ivermectin activates GIRK channels in a PIP2 -dependent, Gβγ -independent manner and an amino acid residue at the slide helix governs the activation. J Physiol (2017). doi: 10.1113/JP274871. PubMed 28715108
- Tortosa E, Adolfs Y, Fukata M, Pasterkamp RJ, Kapitein LC, Hoogenraad CC. Dynamic Palmitoylation Targets MAP6 to the Axon to Promote Microtubule Stabilization during Neuronal Polarization. Neuron 94:809-825 (2017). doi: 10.1016/j.neuron.2017.04.042. PubMed 28521134
- Ogino H, Hisanaga A, Kohno T, Kondo Y, Okumura K, Kamei T, Sato T, Asahara H, Tsuiji H, Fukata M, Hattori M. Secreted Metalloproteinase ADAMTS-3 Inactivates Reelin. J Neurosci. 37:3181-3191 (2017). doi: 10.1523/JNEUROSCI.3632-16.2017. PubMed 28213441
- Uemura T, Shiroshima T, Maeda A, Yasumura M, Shimada T, Fukata Y, Fukata M, Yoshida T. In situ screening for postsynaptic cell adhesion molecules during synapse formation. J Biochem. (2017). doi: 10.1093/jb/mvx030. PubMed 28449070
- Cho T, Ishii-Kato A, Fukata Y, Nakayama Y, Iida K, Fukata M, Iida H. Coupling of a voltage-gated Ca2+ channel homolog with a plasma membrane H+-ATPase in yeast. Genes to Cells 22:94-104 (2017). doi: 10.1111/gtc.12458. PubMed 27935186
- Sugio S, Tohyama K, Oku S, Fujiyoshi K, Yoshimura T, Hikishima K, Yano R, Fukuda T, Nakamura M, Okano H, Watanabe M1, Fukata M, Ikenaka K, F Tanaka K. Astrocyte-mediated infantile-onset leukoencephalopathy mouse model. Glia 65:150-168 (2017). doi: 10.1002/glia.23084. PubMed 27748972
- 2016年
- Fukata Y, Yokoi N, Miyazaki Y, Fukata M. The LGI1-ADAM22 protein complex in synaptic transmission and synaptic disorders. Neurosci Res. 116:39-45 (2016). doi: 10.1016/j.neures.2016.09.011. PubMed 27717669
- Yokoi N*, Fukata Y*, Sekiya A, Murakami T, Kobayashi K, Fukata M. Identification of PSD-95 Depalmitoylating Enzymes. J Neurosci. 36:6431-44 (2016). doi:10.1523/JNEUROSCI.0419-16.2016. PubMed 27307232
- Muona M, Fukata Y, Anttonen AK, Laari A, Palotie A, Pihko H, Lönnqvist T, Valanne L, Somer M, Fukata M, Lehesjoki AE. Dysfunctional ADAM22 implicated in progressive encephalopathy with cortical atrophy and epilepsy. Neurol Genet. 2:e46 (2016). doi: 10.1212/NXG.0000000000000046. PubMed 27066583
- Fukata Y, Murakami T, Yokoi N, Fukata M. Local Palmitoylation Cycles and Specialized Membrane Domain Organization. Curr Top Membr. 77:97-141 (2016). doi: 10.1016/bs.ctm.2015.10.003. PubMed 26781831
- 2015年
- Lovero KL, Fukata Y, Granger AJ, Fukata M, Nicoll RA. The LGI1-ADAM22 protein complex directs synapse maturation through regulation of PSD-95 function. Proc Natl Acad Sci USA. 112:E4129-E4137 (2015). doi: 10.1073/pnas.1511910112. PubMed 26178195
- Yokoi N, Fukata Y, Kase D, Miyazaki T, Jaegle M, Ohkawa T, Takahashi N, Iwanari H, Mochizuki Y, Hamakubo T, Imoto K, Meijer D, Watanabe M, Fukata M. Chemical corrector treatment ameliorates increased seizure susceptibility in a mouse model of familial epilepsy. Nat Med. 21:19-26 (2015). doi:10.1038/nm.3759. PubMed 25485908
- Fukata M, Sekiya A, Murakami T, Yokoi N, Fukata Y. Postsynaptic nanodomains generated by local palmitoylation cycles. Biochem Soc Trans. 43:199-204 (2015). doi: 10.1042/BST20140238. PubMed 25849917
- Suzuki M, Murakami T, Cheng J, Kano H, Fukata M, Fujimoto T. ELMOD2 is anchored to lipid droplets by palmitoylation and regulates adipocyte triglyceride lipase recruitment. Mol Biol Cell. 26:2333-2342 (2015). doi: 10.1091/mbc.E14-11-1504. PubMed 25904333
- Zhu D, Li C, Swanson AM, Villalba RM, Guo J, Zhang Z, Matheny S, Murakami T, Stephenson JR, Daniel S, Fukata M, Hall RA, Olson JJ, Neigh GN, Smith Y, Rainnie DG, Van Meir EG. BAI1 regulates spatial learning and synaptic plasticity in the hippocampus. J Clin Invest. 125:1497-1508 (2015). doi: 10.1172/JCI74603. PubMed 25751059
- 2014年
- Ohkawa T, Satake S, Yokoi N, Miyazaki Y, Ohshita T, Sobue G, Takashima H, Watanabe O, Fukata Y, Fukata M. Identification and characterization of GABA(A) receptor autoantibodies in autoimmune encephalitis. J Neurosci. 34:8151-8163 (2014). doi: 10.1523/JNEUROSCI.4415-13.2014. PubMed 24920620
- Kegel L, Jaegle M, Driegen S, Aunin E, Leslie K, Fukata Y, Watanabe M, Fukata M, Meijer D. Functional phylogenetic analysis of LGI proteins identifies an interaction motif crucial for myelination. Development. 141:1749-1756 (2014). doi: 10.1242/dev.107995. PubMed 24715463
- Gory-Fauré S, Windscheid V, Brocard J, Montessuit S, Tsutsumi R, Denarier E, Fukata Y, Bosc C, Delaroche J, Collomb N, Fukata M, Martinou JC, Pernet-Gallay K, Andrieux A. Non-microtubular localizations of microtubule-associated protein 6 (MAP6). PLoS One. 9:e114905 (2014). doi:10.1371/journal.pone.0114905. PubMed 25526643
- 2013年
- Ohkawa T, Fukata Y, Yamasaki M, Miyazaki T, Yokoi N, Takashima H, Watanabe M, Watanabe O, Fukata M. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J Neurosci 33:18161-18174 (2013) doi: 10.1523/JNEUROSCI.3506-13.2013 PubMed 24227725
- Fukata Y, Dimitrov A, Boncompain G, Vielemeyer O, Perez F, Fukata M. Local palmitoylation cycles define activity-regulated postsynaptic subdomains J Cell Biol 202:145-161 (2013) doi: 10.1083/jcb.201302071 PubMed 23836932
- Oku S, Takahashi N, Fukata Y, Fukata M. In silico screening for palmitoyl substrates reveals a role for DHHC1/3/10 (zDHHC1/3/11)-mediated neurochondrin palmitoylation in its targeting to Rab5-positive endosomes J Biol Chem 288:19816-19829 (2013) doi: 10.1074/jbc.M112.431676 PubMed 23687301
- Zheng B, DeRan M, Li X, Liao X, Fukata M, Wu X. 2-Bromopalmitate analogues as activity-based probes to explore palmitoyl acyltransferases. J Am Chem Soc 135:7082-7085 (2013) doi: 10.1021/ja311416v PubMed 23631516
- Kawahara A, Kurauchi S, Fukata Y, Martínez-Hernández J, Yagihashi T, Itadani Y, Sho R, Kajiyama T, Shinzato N, Narusuye K, Fukata M, Lujテ。n R, Shigemoto R, Ito I. Neuronal major histocompatibility complex class I molecules are implicated in the generation of asymmetries in hippocampal circuitry. J Physiol 591:4777-4791 (2013) PubMed 23878366
- 2012年
- Yokoi N,Fukata M, Fukata Y. Synaptic plasticity regulated by protein-protein interactions and posttranslational modifications. Int Rev Cell Mol Biol. 297:1-43 (2012). doi: 10.1016/B978-0-12-394308-8.00001-7. PubMed 22608556
- Kusuzawa S, Honda T, Fukata Y, Fukata M, Kanatani S, Tanaka DH, Nakajima K. Leucine-rich glioma inactivated 1 (Lgi1), an epilepsy-related secreted protein, has a nuclear localization signal and localizes to both the cytoplasm and the nucleus of the caudal ganglionic eminence neurons. Eur J Neurosci. 36:2284-2292 (2012). doi: 10.1111/j.1460-9568.2012.08129.x. PubMed 22612501
- Lu D, Sun HQ, Wang H, Barylko B, Fukata Y, Fukata M, Albanesi JP, Yin HL. Phosphatidylinositol 4-kinase IIα is palmitoylated by Golgi-localized palmitoyltransferases in cholesterol-dependent manner. J Biol Chem. 287:21856-21865 (2012). doi: 10.1074/jbc.M112.348094. PubMed 22535966
- Oku S, Fukata Y, and Fukata M. DHHC proteins. Encyclopedia of Signaling Molecules. Edited by Choi, S., 1st Edition, Springer (2012). ISBN 978-1-4419-0460-7
- 2011年
- Seppälä EH, Jokinen TS, Fukata M, Fukata Y, Webster MT, Karlsson EK, Kilpinen SK, Steffen F, Dietschi E, Leeb T, Eklund R, Zhao X, Rilstone JJ, Lindblad-Toh K, Minassian BA, Lohi H. LGI2 truncation causes a remitting focal epilepsy in dogs. PLoS Genetics. 7:e1002194 (2011). doi: 10.1371/journal.pgen.1002194. PubMed 21829378
- Levy AD, Devignot V, Fukata Y, Fukata M, Sobel A, Chauvin S. Subcellular Golgi localization of stathmin family proteins is promoted by a specific set of DHHC palmitoyl transferases. Mol Biol Cell. 22:1930-1942 (2011). doi: 10.1091/mbc.E10-10-0824. PubMed 21471001
- 2010年
- Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev Neurosci. 11:161-175 (2010). doi: 10.1038/nrn2788. PubMed 20168314
- Fukata Y, Lovero KL, Iwanaga T, Watanabe A, Yokoi N, Tabuchi K, Shigemoto R, Nicoll RA, Fukata M. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc Natl Acad Sci USA. 107:3799-3804 (2010). doi: 10.1073/pnas.0914537107. PubMed 20133599s
- 2009年
- Noritake J, Fukata Y, Iwanaga T, Hosomi N, Tsutsumi R, Matsuda N, Tani H, Iwanari H, Mochizuki Y, Kodama T, Matsuura Y, Bredt DS, Hamakubo T, Fukata M. Mobile DHHC palmitoylating enzyme mediates activity-sensitive synaptic targeting of PSD-95. J Cell Biol. 186:147-160 (2009). doi: 10.1083/jcb.200903101. PubMed 19596852
- Iwanaga T, Tsutsumi R, Noritake J, Fukata Y, Fukata M. Dynamic protein palmitoylation in cellular signaling. Prog Lipid Res. 48:117-127 (2009). doi: 10.1016/j.plipres.2009.02.001. PubMed 19233228
- Tsutsumi R, Fukata Y, Noritake J, Iwanaga T, Perez F, Fukata M. Identification of G protein alpha subunit-palmitoylating enzyme. Mol Cell Biol. 29:435-447 (2009). doi: 10.1128/MCB.01144-08. PubMed 19001095
- Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, Nemoto T, Fukata M, Poo MM. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci. 29:14185-14198 (2009). doi: 10.1523/JNEUROSCI.1863-09. PubMed 19906967
- Shmueli A, Segal M, Sapir T, Tsutsumi R, Noritake J, Bar A, Sapoznik S. Fukata Y, Orr I Fukata M, Reiner O. Ndel1 palmitoylation: a new mean to regulate cytoplasmic dynein activity. EMBO J. 29:107-119 (2010). doi: 10.1038/emboj.2009.325. PubMed 19927128
- Mill P, Lee AW, Fukata Y, Tsutsumi R, Fukata M, Keighren M, Porter RM, McKie L, Smyth I, Jackson IJ. Palmitoylation regulates epidermal homeostasis and hair follicle differentiation. PLoS Genet. 5:e1000748 (2009). doi: 10.1371/journal.pgen.1000748. PubMed 19956733
- Vetrivel KS, Meckler X, Chen Y, Nguyen PD, Seidah NG, Vassar R, Wong PC, Fukata M, Kounnas MZ, Thinakaran G. Alzheimer disease Abeta production in the absence of S-palmitoylation-dependent targeting of BACE1 to lipid rafts. J Biol Chem. 284:3793-3803 (2009). doi: 10.1074/jbc.M808920200. PubMed 19074428
- Greaves J, Prescott GR, Fukata Y, Fukata M, Salaun C, Chamberlain LH. The hydrophobic cysteine-rich domain of SNAP25 couples with downstream residues to mediated membrane interactions and recognitions by DHHC palmitoyl transferases. Mol Biol Cell. 20:1845-1854 (2009). doi: 10.1091/mbc.E08-09-0944. PubMed 19158383
- 2008年
- Ponimaskin E, Dityateva G, Ruonala MO, Fukata M, Fukata Y, Kobe F, Wouters FS, Delling M, Bredt DS, Schachner M, Dityatev A. Fibroblast growth factor-regulated palmitoylation of the neural cell adhesion molecule determines neuronal morphogenesis. J Neurosci. 28:8897-8907 (2008). doi: 10.1523/JNEUROSCI.2171-08. PubMed 18768683
- Greaves J, Salaun C, Fukata Y, Fukata M, Chamberlain LH. Palmitoylation and membrane interactions of the neuroprotective chaperone cysteine-string protein. J Biol Chem. 283:25014-25026 (2008). doi: 10.1074/jbc.M802140200. PubMed 18596047
- Tsutsumi R, Fukata Y, Fukata M. Discovery of protein-palmitoylating enzymes. Pflugers Arch. 456:1199-1206 (2008). doi: 10.1007/s00424-008-0465-x. PubMed 18231805
- 2007年
- Olsen O, Funke L, Long JF, Fukata M, Kazuta T, Trinidad JC, Moore KA, Misawa H, Welling PA, Burlingame AL, Zhang M, Bredt DS. Renal defects associated with improper polarization of the CRB and DLG polarity complexes in MALS-3 knockout mice. J Cell Biol 179:151-164 (2007) PubMed 17923534
- Mishima M, Maesaki R, Kasa M, Watanabe T, Fukata M, Kaibuchi K, Hakoshima T. Structural basis for tubulin recognition by cytoplasmic linker protein 170 and its autoinhibition. Proc Natl Acad Sci USA. 104:10346-10351 (2007). doi: 10.1073/pnas.0703876104. PubMed 17563362
- Yamamoto N, Fukata Y, Fukata M, Yanagisawa K. GM1-ganglioside-induced Abeta assembly on synaptic membranes of cultured neurons. Biochim Biophys Acta. 1768:1128-1137 (2007). doi: 10.1016/j.bbamem.2007.01.009. PubMed 17306220
- Wang S, Watanabe T, Noritake J, Fukata M, Yoshimura T, Itoh N, Harada T, Nakagawa M, Matsuura Y, Arimura N, Kaibuchi K. IQGAP3, a novel effector of Rac1 and Cdc42, regulates neurite outgrowth. J Cell Sci. 120:567-577 (2007). doi: 10.1242/jcs.03356. PubMed 17244649
- Tomita S, Shenoy A, Fukata Y, Nicoll RA, Bredt DS. Stargazin interacts functionally with the AMPA receptor glutamate-binding module. Neuropharmacology. 52:87-91 (2007). doi: 10.1016/j.neuropharm.2006.07.012. PubMed 16919685
- 2006年
- Fukata Y, Adesnik H, Iwanaga T, Bredt DS, Nicoll RA, Fukata M. Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science 313:1792-1795 (2006) PubMed 16990550
- Fukata Y, Iwanaga T, Fukata M. Systematic screening for palmitoyl transferase activity of the DHHC protein family in mammalian cells. Methods 40:177-182 (2006) PubMed 17012030
- Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Lüscher B. GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci. 26:12758-12768 (2006). doi: 10.1523/JNEUROSCI.4214-06.2006. PubMed 17151279
- Fernández-Hernando C, Fukata M, Bernatchez PN, Fukata Y, Lin MI, Bredt DS, Sessa WC. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J Cell Biol. 174:369-377 (2006). doi: 10.1083/jcb.200601051. PubMed 16864653
- Hundt M, Tabata H, Jeon MS, Hayashi K, Tanaka Y, Krishna R, De Giorgio L, Liu YC, Fukata M, Altman A. Impaired activation and localization of LAT in anergic T cells as a consequence of a selective palmitoylation defect. Immunity. 24:513-522 (2006). doi: 10.1016/j.immuni.2006.03.011. PubMed 16713970
- Fukata Y, Bredt DS, Fukata M. Protein palmitoylation by DHHC protein family. The CRC Press: The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology. 81-88 (2006). PubMed 81-88
- 2005年
- Fukata Y, Tzingounis AV, Trinidad JC, Fukata M, Burlingame AL, Nicoll RA, Bredt DS. Molecular constituents of neuronal AMPA receptor. J Cell Biol. 169:399-404 (2005). doi: 10.1083/jcb.200501121. PubMed 15883194
- Olsen O, Moore KA, Fukata M, Kazuta T, Trinidad J, Kauer FW, Streuli M, Misawa H, Burlingame AL, Nicoll RA, Bredt DS. Neurotransmitter release regulated by a MALS-liprin-a presynaptic complex. J Cell Biol. 170:1127-1134 (2005). doi: 10.1083/jcb.200503011. PubMed PMC2171538
- Arimura N, Ménager C, Kawano Y, Yoshimura T, Kawabata S, Hattori A, Fukata Y, Amano M, Goshima Y, Inagaki M, Morone N, Usukura J, Kaibuchi K. Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Mol Cell Biol. 25: 9973-9984 (2005). doi: 10.1128/MCB.25.22.9973-9984.2005. PubMed 16260611
- Kimura T, Arimura N, Fukata Y, Watanabe H, Iwamatsu A, Kaibuchi K. Tubulin and CRMP-2 complex is transported via Kinesin-1. J Neurochem. 93: 1371-1382 (2005). doi: 10.1111/j.1471-4159.2005.03063.x. PubMed 15935053
- 2004年
- Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 Palmitoylating Enzymes. Neuron. 44:987-996 (2004). doi: 10.1016/j.neuron.2004.12.005. PubMed 15603741
- Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 6:871-883 (2004). doi: 10.1016/j.devcel.2004.10.017. PubMed 15572129
- Tomita S, Fukata M, Nicoll RA, Bredt DS. Dynamic interaction of stargazin-like TARPs with cycling AMPA receptors at synapses. Science. 303:1508-1511 (2004). doi: 10.1126/science.1090262. PubMed 15001777
- Noritake J, Fukata M, Sato K, Nakagawa M, Watanabe T, Izumi N, Wang S, Fukata Y, Kaibuchi K. Positive Role of IQGAP1, an Effector of Rac1, in Actin-Meshwork Formation at Sites of Cell-Cell Contact. Mol Biol Cell. 3:1065-1076 (2004). doi: 10.1091/mbc.e03-08-0582. PubMed 14699063
- Mélnager C, Arimura N, Fukata Y, Kaibuchi K. PIP3 is involved in neuronal polarization and axon formation. J Neurochem 89:109-118 (2004) PubMed 15030394
- Arimura N, Menager C, Fukata Y, Kaibuchi K. Role of CRMP-2 in neuronal polarity. J Neurobiol. 58:34-47 (2004). doi: 10.1002/neu.10269. PubMed 14598368
- Nishimura T, Kato K, Yamaguchi T, Fukata Y, Ohno S, Kaibuchi K. Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat Cell Biol. 6:328-334 (2004). doi: 10.1038/ncb1118. PubMed 15048131
- 2003年
- Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration Curr Opin Cell Biol. 15:590-597 (2003). doi: 10.1016/s0955-0674(03)00097-8. PubMed 14519394
- Amano M, Kaneko T, Maeda A, Nakayama M, Ito M, Yamauchi T, Goto H, Fukata Y, Oshiro N, Shinohara A, Iwamatsu A, Kaibuchi K. Identification of Tau and MAP2 as novel substrates of Rho-kinase and myosin phosphatase. J Neurochem. 87:780-790 (2003). doi: 10.1046/j.1471-4159.2003.02054.x. PubMed 14535960
- Nishimura T, Fukata Y, Kato K, Yamaguchi T, Matsuura Y, Kamiguchi H, Kaibuchi K. CRMP-2 regulates polarized Numb-mediated endocytosis for axon growth. Nat Cell Biol. 5:819-826 (2003). doi: 10.1038/ncb1039. PubMed 12942088
- 2002年
- Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K. Rac1 and Cdc42 Capture Microtubules through IQGAP1 and CLIP-170. Cell. 109:873-885 (2002). doi: 10.1016/s0092-8674(02)00800-0. PubMed 12110184
- Fukata M, Nakagawa M, Kuroda S, Kaibuchi K. Effects of Rho family GTPases on cell-cell adhesion. Methods Mol Biol. 189:121-128 (2002). doi: 10.1385/1-59259-281-3:121. PubMed 12094580
- Fukata Y, Itoh TJ, Kimura T, Ménager C, Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani H, Kaibuchi K. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol. 4:583-591 (2002). doi: 10.1038/ncb825. PubMed 12134159
- Fukata Y, Kimura T, Kaibuchi K. Axon specification in hippocampal neurons. Neurosci Res. 43: 305-315 (2002). doi: 10.1016/s0168-0102(02)00062-7. PubMed 12135774
- Maeda A, Amano M, Fukata Y, Kaibuchi K. Translocation of Na(+),K(+)-ATPase is induced by Rho small GTPase in renal epithelial cells. Biochem Biophys Res Commun. 297:1231-1237 (2002). doi: 10.1016/s0006-291x(02)02342-2. PubMed 12372419
- 2001年
- Fukata M, Kaibuchi K. Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nature Reviews Mol Cell Biol 12:887-897 (2001) PubMed 11733768
- Fukata M, Nakagawa M, Itoh N, Kawajiri A, Yamaga M, Kuroda S, Kaibuchi K. Involvement of IQGAP1, an effector of Rac1 and Cdc42 GTPases, in cell-cell dissociation during cell scattering. Mol Cell Biol. 21:2165-2183 (2001). doi: 10.1128/MCB.21.6.2165-2183.2001. PubMed 11238950
- Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trend Pharmacol Sci. 22:32-39 (2001). doi: 10.1016/s0165-6147(00)01596-0. PubMed 11165670
- Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K. Recruitment and activation of Rac1 by the formation of E-cadherin- mediated cell-cell adhesion sites. J Cell Sci. 114:1829-1838 (2001). doi: 10.1242/jcs.114.10.1829. PubMed 11329369
- 2000年
- Amano M, Fukata Y, Shimokawa H, Kaibuchi K. Purification and in Vitro Activity of Rho-Associated Kinase. Methods Enzymol 325:149-155 (2000) PubMed 11036600
- Amano M, Fukata Y, Kaibuchi K. Regulation and Functions of Rho-Associated Kinase. Exp Cell Res. 261:44-51 (2000). doi: 10.1006/excr.2000.5046. PubMed 11082274
- Izawa T, Fukata Y, Kimura T, Iwamatsu A, Dohi K, Kaibuchi K. Elongation factor-1 alpha is a novel substrate of rho-associated kinase. Biochem Biophys Res Commun. 278:72-78 (2000). doi: 10.1006/bbrc.2000.3772. PubMed 11071857
- Kawajiri A, Itoh N, Fukata M, Nakagawa M, Yamaga M, Iwamatsu A, Kaibuchi K. Identification of a novel beta-catenin-interacting protein. Biochem Biophys Res Commun. 273:712-717 (2000). doi: 10.1006/bbrc.2000.3002. PubMed 10873669
- Nakamura N, Oshiro N, Fukata Y, Amano M, Fukata M, Kuroda S, Matsuura Y, Leung T, Lim L, Kaibuchi K. Phosphorylation of ERM proteins at filopodia induced by Cdc42. Genes Cells. 5:571-581 (2000). doi: 10.1046/j.1365-2443.2000.00348.x. PubMed 10947843
- Sone M, Suzuki E, Hoshino M, Hou D, Kuromi H, Fukata M, Kuroda S, Kaibuchi K, Nabeshima Y, Hama C. Synaptic development is controlled in the periactive zones of Drosophila synapses. Development. 127:4157-4168 (2000). doi: 10.1242/dev.127.19.4157. PubMed 10976048
- Kandabashi T, Shimokawa H, Miyata K, Kunihiro I, Kawano Y, Fukata Y, Higo T, Egashira K, Takahashi S, Kaibuchi K, Takeshita A. Inhibition of myosin phosphatase by upregulated rho-kinase plays a key role for coronary artery spasm in a porcine model with interleukin-1beta. Circulation. 101:1319-1323 (2000). doi: 10.1161/01.cir.101.11.1319. PubMed 10725293
- 1999年
- Fukata M, Nakagawa M, Kuroda S, Kaibuchi K. Cell adhesion and Rho small GTPases. J Cell Sci 112:4491-4500 (1999) PubMed 10574699
- Fukata M, Kuroda S, Nakagawa M, Kawajiri A, Itoh N, Shoji I, Matsuura Y, Yonehara S, Fujisawa H, Kikuchi A, Kaibuchi K. Cdc42 and Rac1 regulate the interaction of IQGAP1 with beta-catenin. J Biol Chem. 274:26044-26050 (1999). doi: 10.1074/jbc.274.37.26044. PubMed 10473551
- Fukata Y, Oshiro N, Kinoshita N, Kawano Y, Matsuoka Y, Bennett V, Matsuura Y, Kaibuchi K. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J Cell Biol. 145:347-361 (1999). doi: 10.1083/jcb.145.2.347. PubMed 10209029
- Fukata Y, Oshiro N, Kaibuchi K. Activation of moesin and adducin by Rho-kinase downstream of Rho. Biophys Chem 82:139-147 (1999) PubMed 10631797
- Kuroda S, Fukata M, Nakagawa M, Kaibuchi K. Cdc42, Rac1, and their effector IQGAP1 as molecular switches for cadherin-mediated cell-cell adhesion. Biochem Biophys Res Commun. 262:1-6 (1999). doi: 10.1006/bbrc.1999.1122. PubMed 10448058
- Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 147:1023-1038 (1999). doi: 10.1083/jcb.147.5.1023. PubMed 10579722
- Hoshino M, Sone M, Fukata M, Kuroda S, Kaibuchi K, Nabeshima Y, Hama C. Identification of the stef gene that encodes a novel guanine nucleotide exchange factor specific for Rac1. J Biol Chem. 274:17837-17844 (1999). doi: 10.1074/jbc.274.25.17837. PubMed 10364228
- Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr Opin Cell Biol. 11:591-596 (1999). doi: 10.1016/s0955-0674(99)00014-9. PubMed 10508646
- 1998年
- Fukata Y, Kimura K, Oshiro N, Saya H, Matsuura Y, Kaibuchi K. Association of the myosin-binding subunit of myosin phosphatase and moesin: dual regulation of moesin phosphorylation by Rho-kinase and myosin phosphatase. J Cell Biol. 141:409-418 (1998). doi: 10.1083/jcb.141.2.409. PubMed 9548719
- Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, Shoji I, Matsuura Y, Yonehara S, Kaibuchi K. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell-cell adhesion. Science. 281:832-835 (1998). doi: 10.1126/science.281.5378.832. PubMed 9694656
- Kimura K, Fukata Y, Matsuoka Y, Bennett V, Matsuura Y, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of the association of adducin with actin filaments by Rho-kinase and myosin phosphatase. J Biol Chem. 273:5542-5548 (1998). doi: 10.1074/jbc.273.10.5542. PubMed 9488679
- Oshiro N, Fukata Y, Kaibuchi K. Phosphorylation of Moesin by Rho-associated Kinase (Rho-kinase) Plays a Crucial Role in the Formation of Microvilli-like Structures. J Biol Chem. 273:34663-34666 (1998). doi: 10.1074/jbc.273.52.34663. PubMed 9856983
- Amano M, Fukata Y, Kaibuchi K. Regulation of Cytoskeleton and Cell Adhesions by the Small GTPase Rho and Its Targets. Trends Cardiovasc Med. 8:162-168(1998). doi: 10.1016/S1050-1738(97)00145-X. PubMed 21235928
- Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, Ikebe M, Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 3:177-188 (1998). doi: 10.1046/j.1365-2443.1998.00181.x. PubMed 9619630
- Kobayashi K, Kuroda S, Fukata M, Nakamura T, Nagase T, Nomura N, Matsuura Y, Yoshida-Kubomura N, Iwamatsu A, Kaibuchi K. p140Sra-1 (specifically Rac1-associated protein) is a novel specific target for Rac1 small GTPase. J Biol Chem. 273:291-295 (1998). doi: 10.1074/jbc.273.1.291. PubMed 9417078
- 1997年
- Fukata M, Kuroda S, Fujii K, Nakamura T, Shoji I, Matsuura Y, Okawa K, Iwamatsu A, Kikuchi A, Kaibuchi K. Regulation of cross-linking of actin filament by IQGAP1, a target for Cdc42. J Biol Chem. 272:29579-29583 (1997). doi: 10.1074/jbc.272.47.29579. PubMed 9368021
- Kuroda S, Fukata M, Fujii K, Nakamura T, Izawa I, Kaibuchi K. Regulation of cell-cell adhesion of MDCK cells by Cdc42 and Rac1 small GTPases. Biochem Biophys Res Commun. 240:430-435 (1997). doi: 10.1006/bbrc.1997.7675. PubMed 9388496
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions by Rho-kinase. Science. 275:1308-1311 (1997). doi: 10.1126/science.275.5304.1308. PubMed 9036856
- 1996年
- Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J Biol Chem. 271:23363-23367 (1996). doi: 10.1074/jbc.271.38.23363. PubMed 8798539
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 273:245-248 (1996). doi: 10.1126/science.273.5272.245. PubMed 8662509
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem. 271:20246-20249 (1996). doi: 10.1074/jbc.271.34.20246. PubMed 8702756
研究キーワード
シナプス生物学、シナプス病態、パルミトイル化脂質修飾、生化学、超解像イメージング、てんかん、知的障害、自己免疫性脳炎、AMPA受容体、LGI1-ADAM22
大学院生募集
- 共に興味を分かち合い、世界に情報発信したいと望む若者を募集しています。
- 研究室メンバーのバックグランドは、医学、理学、薬学、農学と多彩です。
- 興味のある方は、まずはお気軽に連絡をください。