NEWS&EVENT
- Back
- Top > NEWS&EVENT > Nagoya University and Chulalongkorn University, Collaboration in Thailand -Successful treatment of malignant lymphoma using non-viral vector-based CAR-T cells-
Nagoya University and Chulalongkorn University, Collaboration in Thailand -Successful treatment of malignant lymphoma using non-viral vector-based CAR-T cells-
Summary
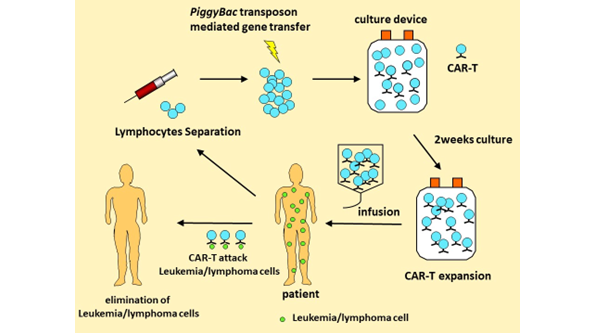
A research team led by Professor Yoshiyuki Takahashi in the Department of Pediatrics, Nagoya University Graduate School of Medicine and designated lecturer Nobuhiro Nishio in the Department of Advanced Medicine have developed a chimeric antigen receptor (*2) gene-modified T cell (CAR-T cell) therapy employing the piggyBac transposon method (*1). In December 2018, the Faculty of Medicine, Nagoya University signed a material transfer agreement to support the use of CAR-T cell therapy with the Faculty of Medicine, Chulalongkorn University, Thailand that has been supporting the clinical research with CAR-T cells in Thailand. A news conference will be held at Chulalongkorn University to announce that CAR-T cell therapy was effective in all the five patients with relapsed and refractory malignant lymphoma. Unlike Europe, USA, and Japan, there are no approved CAR-T cell products in Thailand; therefore, the availability of CAR-T cell therapy at Chulalongkorn University has an important social impact in Thailand.
For this achievement, two technicians from Chulalongkorn University returned to Thailand after receiving technical assistance at Nagoya University, and the clinical trials for acute lymphoblastic leukemia (ALL) and malignant lymphomas have commenced since 2020.
In 2023, Nagoya University also plans to start an investigator-initiated clinical trial utilizing CAR-T cells for relapsed/refractory malignant lymphomas. In the Japanese clinical trials, CD19.CAR-T cells (code name: JPCAR019) manufactured by the Japan Tissue Engineering Corporation (J-TEC/Teijin group), with whom we have signed a license agreement regarding the patented technology for this cell preparation, will be employed in the clinical trials at Hokkaido University Hospital, National Cancer Center Hospital East, Nagoya University Hospital, Kyoto University Hospital, and Kyushu University Hospital.
Key Points
• In a clinical study, using CAR-T cells, conducted at Chulalongkorn University, Thailand and supported by Nagoya University, five patients with malignant lymphoma have received the treatment that has been effective and a clinical trial is ongoing without any safety concerns.
• Nagoya University Hospital will begin an investigator-initiated clinical trial this year using CD19.CAR-T cells (code name: JPCAR019) manufactured by Japan Tissue Engineering Corporation (J-TEC/Teijin group), which has signed a license agreement for the patented technology related to this cell preparation.
Background
Acute lymphocytic leukemia (ALL) and malignant lymphomas are disorders that occur when lymphocytes in the blood become "cancerous" and reproduce indefinitely in the patient's body. Recently, chimeric antigen receptor (CAR)-T cell therapies have been developed for relapsed or refractory ALL and malignant lymphomas, and have shown higher response rates than conventional therapies. CARs are proteins having a structure that connects the antigen recognition site of an antibody to the intracellular signaling domain of the T cell receptor. CAR-T cell therapy is a treatment in which T cells are transferred with the CAR gene in vitro and infused back into the patient. The production of CAR-T cells, including products approved in Japan and abroad, employs viral vectors such as retroviruses and lentiviruses for gene transfer, but the production costs are extremely high.
The research group has developed a method for producing CAR-T cells by gene transfer using the piggyBac transposon, a non-viral vector, and has initiated a clinical trial. This method uses an enzyme, which is simpler and can be manufactured at a lower cost than viral vectors. Furthermore, it is expected to have the same therapeutic effect as viral vectors.
In 2018, Chulalongkorn University Hospital in Thailand requested Nagoya University and signed an agreement to provide support for clinical trials using the same protocol of Nagoya University. Two technicians from Chulalongkorn University returned to Thailand after receiving technical assistance at Nagoya University, and clinical trials for ALL and malignant lymphomas have started since 2020.
Results
PiggyBac CAR-T cell therapy was safely administered to patients with malignant lymphoma for the first time in Thailand, and the clinical trial is ongoing. Five patients with malignant lymphoma were treated so far, and a therapeutic response has been observed in all patients. One patient with a large abdominal mass maintained a complete metabolic response one year after treatment.
Future Perspective
As part of the international collaboration, we will continue to support the implementation of CAR-T cell therapy using this production method in Southeast Asian countries, including Thailand, where it is challenging to produce CAR-T cells using viral vectors because of regulatory and cost issues.
Furthermore, our goal is to ensure this therapy is covered by the health insurance companies to make it available to as many patients as possible. J-TEC signed a license agreement with Nagoya University and Shinshu University in June 2018 to introduce this technology and is preparing to start clinical trials of CD19.CAR-T cells (code name: JPCAR019) for ALL. Moreover, an investigator-initiated clinical trial utilizing JPCAR019 for malignant lymphoma will soon be initiated. Furthermore, by modifying the antigen recognition site of CAR, our aim is to apply the CAR-T cell product to other solid tumors.
This research was supported by the following:
FY2015-2016 Research for Practical Research for Innovative Cancer Control, Japan Agency for Medical Research and Development (AMED): "Development of chimeric antigen receptor T cell therapy using non-viral vectors for pediatric acute lymphocytic leukemia" (Principal investigator: Yoshiyuki Takahashi, Professor, Department of Pediatrics, Nagoya University Graduate School of Medicine).
FY2017-2019 Research for Practical Research for Innovative Cancer Control, Japan Agency for Medical Research and Development (AMED): "Establishment of Chimeric Antigen Receptor T cell therapy for Acute Lymphoblastic Leukemia using Non-Virus Vector system" (Principal investigator: Yoshiyuki Takahashi, Professor, Department of Pediatrics, Nagoya University Graduate School of Medicine).
FY2020-2022 Research for Practical Research for Innovative Cancer Control, Japan Agency for Medical Research and Development (AMED): "Phase Ⅰ/Ⅱ investigator-initiated clinical trial of piggyBac transposon-mediated chimeric antigen receptor gene-modified T cells for CD19-positive malignant lymphoma" (Principal Investigator: Yoshiyuki Takahashi, Professor, Department of Pediatrics, Nagoya University Graduate School of Medicine).