Laboratories
- Back
- Top > Laboratories > Molecular Pharmacology > Toxicogenomics
Molecular PharmacologyToxicogenomics
Introduction
Drug discovery research to enhance clinical drug safety
The Division of Clinical Pharmacology was added to the Division of Basic Medicine and the Division of Clinical Medicine during organizational reform at Nagoya University Graduate School of Medicine in FY2013. Nagoya University has no Faculty of Pharmacy and so the Division of Clinical Pharmacology plays an important role in drug discovery and nurturing researchers in this field. Specialists in chemotherapy, medical pharmacists, and biostatisticians adopt an almost clinical approach to both education and research. Researchers from a broad range of disciplines from basic sciences to clinical medicine work in cooperation with pharmaceutical companies to hold collaborative lectures between academia and industry as our department focuses on solving those medical issues that greatly impact society.
Our Toxicogenomics laboratory in the Division of Clinical Pharmacology, is one of the few laboratories in Japan which specialize in drug safety sciences. Drugs are a double-edged sword with both beneficial and toxic effects. They must be proven safe before they are approved for use, but despite such precautions, patients continue to suffer many adverse effects. Although detailed clinical studies must be conducted before the authorities will approve a drug for marketing, these studies only involve about 3,000 patients at most. However, there are large interindividual differences in drug reactions, and a rare reaction that only occurs in 1 out of 10,000 people cannot possibly be predicted. Despite preclinical studies in animal and cell-based models, some adverse reactions will only occur idiopathically in humans, and they may often become apparent during clinical trials. To avoid drug toxicities despite such individual and species-based differences, our Toxicogenomics laboratory is attempting to elucidate the mysterious mechanisms behind adverse reactions.
Predicting liver injury before clinical studies
The focus of our research is drug-induced liver injury. Over half of all drugs on the market carry label warnings against liver injury, and adverse drug reactions occur in as many as 1 out of 5000-6000 patients. The liver is itself responsible for drug detoxification, but occasionally, it produces reactive metabolites that can cause harmful effects not only in the liver, but also other organs such as the kidneys or skin. To prevent such toxicities, we studied the mechanism of onset and identified factors related to immune function and inflammation. This has allowed us to build a test model that can detect drugs with a potential for liver injury during preclinical studies before they are ever administered to humans. Recently, we developed an animal model of drug-induced myolysis, the breakdown of muscle cells. To make further strides in research on the causes of adverse drug reactions, it is vital that we conduct interventional clinical studies with full respect for the integrity of patient rights and ensuring patient safety. Towards these objectives, we plan to conduct first-in-human clinical studies at our new Advanced Medical Care Facility (working title), and believe that this will lead to major strides in drug discovery. Patients who are prescribed drugs from multiple physicians require proactive care measures and should receive essential information about their treatment to prevent potential drug interactions. Thus, at the Division of Clinical Pharmacology, we feel we must strengthen our comprehensive role in tying together basic and clinical sciences that cover both medical and pharmaceutical disciplines. Our ultimate goal is to produce research results that will directly benefit both patients and society.
Research Projects
1. New prospective and understanding in idiosyncratic drug-induced liver injury (IDILI) considering drug metabolism and immune- and inflammatory-related factors: Establishment of a comprehensive in vivo rodent screening system for the risk assessment of IDILI
IDILI is an important issue for drug development and clinical drug therapy; however, in most cases, it is difficult to predict or prevent these adverse reactions due to a lack of animal models and the unknown mechanisms of action. Many in vitro prediction methods have been reported till now, however, no method can predict the risk of IDILI properly. We believe that the in vivo animal models for IDILI is necessarily to elucidate the underlying mechanisms relating metabolic activation reactions and immune- and inflammatory-related factors, and then to develop the in vitro prediction system. Based on these perspective, IDILI mouse models have been established firstly by our group and elucidated the involving factors in carbamazepine, phenytoin, halothane, diclofenac, flucloxacillin, methimazole, flutamide, dicloxacillin, ANIT (alpha-naphthylisothiocyanate), and azathioprine using normal mice. The underlying mechanisms of IDILI differ in each drug. However, metabolic activation reactions were involved in the onset of IDILI in most drugs. Th17 cells were involved in IDILI drugs of carbamazepine, phenytoin, halothane, diclofenac, and flucloxacillin in the onset and exacerbation of pathogenesis. On the other hand, Th2 cells were involved in IDILI drugs of methimazole, flutamide, and dicloxacillin. Interestingly, Th1 and Treg cells were not involved functionally. Reactive oxygen species (ROS) and other factors were suggested in ANIT and azathioprine (Those drugs are not categorized in IDILI). In addition, chimeric mice with a humanized liver were also investigated as an animal model of troglitazone-induced liver injury. We are accumulating much knowledge of the IDILI rodent models to predict human IDILI.
The future prospects of our project are as follows, (1) IDILI mouse models for some drugs are now on the way to establish using normal mouse. (2) We would like to propose the in vivo mouse screening system that can be used comprehensively in preclinical step for the risk assessment of IDILI
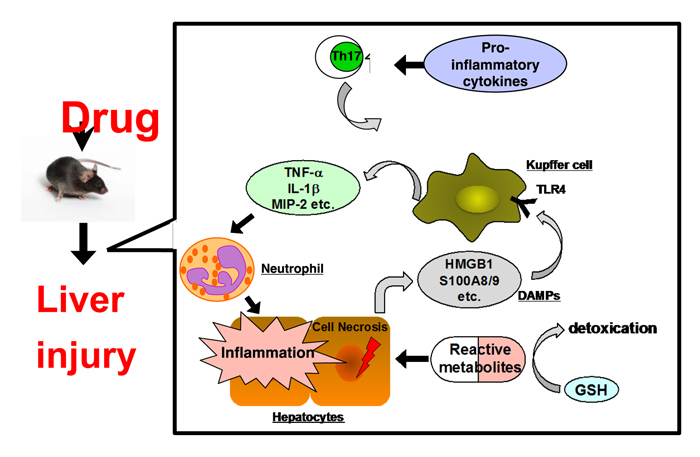
2. A novel cell-based assay for DILI potential considering immune- and inflammatory-related factors
Drug-induced liver injury (DILI) is one of the leading causes of failure in drug development and post-marketing drug withdrawal. Although some preclinical in vitro evaluation of hepatotoxic potential of drugs by utilizing hepatic cell death or cellular stress as markers have been developed, their predictive ability is not efficient. To establish a cell-based system for the assessment of DILI, it is necessary to better understand its mechanism including alterations in gene expression using animal models of DILI.
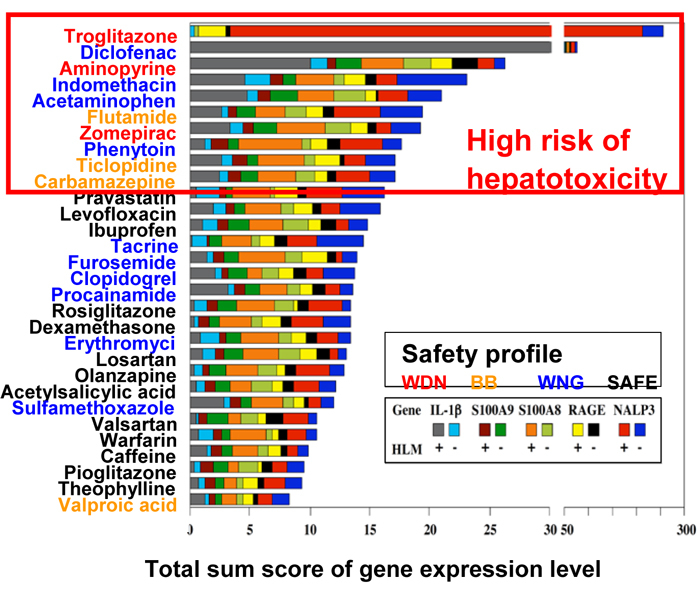
Using in vivo mouse DILI models of hepatotoxic (acetaminophen, dicloxacillin, flutamide, halothane, and diclofenac) and non-hepatotoxic (ampicillin, bicalutamide, isoflurane, and ibuprofen) drugs, we found that the hepatic mRNA levels of immune- and inflammatory-related factors including S100 calcium-binding protein A8 (S100A8), S100A9, “NATCH, LRR, and pyrin domain-containing protein 3” (NLRP3), interleukin (IL)-1beta, and the receptor for advanced glycation endproducts (RAGE) were commonly increased in hepatotoxic drug-administered mice compared to non-hepatotoxic drug-administered mice. To clarify whether these five biomarkers can be applied to a cell-based screening system, human monocytic leukemia cells HL-60, K562, KG-1 and THP-1 were treated with 30 drugs in the presence of human liver microsomes (HLM) and NADPH for considering metabolic activation. We found that the total sum score of gene expression levels of S100A8, S100A9, RAGE, and IL-1beta mRNA in HL-60 cells and NLRP3 mRNA in K562 cells incubated with and without HLM, could identify hepatotoxic drugs. These results raised the possibility that an in vitro assay considering immune- and inflammatory-related factors could improve the prediction of clinical DILI (Yano et al., Toxicol Lett 228: 13-24, 2014).
We next applied human liver carcinoma HepaRG cells that express drug-metabolizing enzymes comparable to primary human hepatocytes to a cell-based assay. HepaRG or HepG2 cells were treated with 96 drugs with different DILI risks and the cultured media were collected. HL-60 cells were subsequently treated with the collected drug-conditioned media and mRNA expression levels of S100A9, IL-1beta, monocyte chemoattractant protein-1 (MCP-1), IL-8, and tumor necrosis factor (TNF) a were investigated. An area under the receiver operating characteristic curve (ROC-AUC) was calculated to evaluate the predictive performance of the mRNA levels as markers to discriminate DILI risks. The expression of IL-8 in HL-60 cells treated with conditioned media from HepaRG cells (HL-60/HepaRG) exhibited the highest ROC-AUC value of 0.758, followed by the expression of IL-1beta in HL-60/HepaRG (ROC-AUC: 0.726). Notably, ROC-AUC values of these genes were higher in HL-60/HepaRG than in HL-60/HepG2, which suggests that HL-60/HepaRG has a higher potential for detecting hepatotoxic drugs. An integrated score calculated from the levels of S100A9, IL-1beta, and IL-8 more precisely determined DILI risks than individual gene expression did. The developed cell-based assay that utilizes immune and inflammatory factors expression would aid in the assessment of potential DILI risks. Collectively, we developed a novel cell-based assay system for risk assessment of DILI and suggested that this assay has a potential utility in screening for DILI in preclinical drug development. Our current projects regarding in vitro assessment of DILI are as follows: (1) to know whether reactive metabolites of hepatotoxic drugs are associated with immune activation; (2) to apply induced pluripotent stem cells (iPS) cells for the assessment of DILI.
Bibliography
- 2017
- Sho Akai, Shingo Oda, and Tsuyoshi Yokoi. Establishment of a novel mouse model for pioglitazone-induced skeletal muscle injury. Toxicology, 2017; 382:1-9.
- Akira Nakajima, Hiroki Sato, Shingo Oda, and Tsuyoshi Yokoi. Fluoroquinolones and propionic acid derivatives induce inflammatory responses in vitro. Cell Biol Toxicol, in press.
- Yuji Shirai, Shingo Oda, Sayaka Makino, Koichi Tsuneyama, and Tsuyoshi Yokoi. Establishment of a mouse model of enalapril-induced liver injury and investigation of the pathogenesis. Lab Invest, in press.
- Shingo Oda, Yuji Shirai, Sho Akai, Akira Nakajima, Koichi Tsuneyama, and Tsuyoshi Yokoi. Toxicological role of an acyl glucuronide metabolite in diclofenac-induced acute liver injury in mice. J Appl Toxicol, 2017; 37:545-553.
- Takafumi Tomida, Hayao Okamura, Tsuyoshi Yokoi, and Yoshihiro Konno. A modified multiparametric assay using HepaRG cells for predicting the degree of drug-induced liver injury risk. J Appl Toxicol, 2017; 37:382-390.
- 横井 毅、織田進吾. 薬物代謝・薬物動態研究の最近の動向と展望 -医薬品開発研究を中心として-。化学と生物, in press.
- Atsushi Iwamura, Miki Nakajima, Shingo Oda, and Tsuyoshi Yokoi. Toxicological potential of acyl glucuronides and its assessment. Drug Metab Pharmacokinet, 2017; 32:2-11.
- 横井 毅. 薬剤性肝胆膵障害「図解 薬害副作用学 改訂2版」川西正祐、小野秀樹、賀川義之編、南山堂、in press.
- 2016
- Uematsu Y, Akai S, Tochitani T, Oda S, Yamada T, Yokoi T. MicroRNA-mediated Th2 bias in methimazole-induced acute liver injury in mice. Toxicol Appl Pharmacol, 2016; 307: 1-9.
- Iwamura A, Watanabe K, Akai S, Nishinosono T, Tsuneyama K, Oda S, Kume T, Yokoi T. Zomepirac acyl glucuronide is responsible for zomepirac-induced acute kidney injury in mice. Drug Metab Dispos, 2016; 44: 888-896.
- Chiangsom A, Lawanprasert S, Oda S, Kulthong K, Luechapudiporn R, Yokoi T, Maniratanachote R. Inhibitory and inductive effects of Phikud Navakot extract on human cytochrome P450. Drug Metab Pharmacokinet, 2016; 31: 210-217.
- Oda S, Matsuo K, Nakajima A, Yokoi T. A novel cell-based assay for the evaluation of immune- and inflammatory-related gene expression as biomarkers for the risk assessment of drug-induced liver injury. Toxicol Lett, 2016; 241: 60-70.
- Nakajima A, Oda S, Yokoi T. Allopurinol induces innate immune responses through mitogen-activated protein kinase signaling pathways in HL-60 cells. J Appl Toxicol, 2016; 36: 1120-1128.
- Sasaki E, Iida A, Oda S, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Pathogenetic analyses of carbamazepine-induced liver injury in F344 rats focused on immune- and inflammation-related factors. Exp Toxicol Pathol, 2016; 68: 27-38.
- Akai S, Uematsu Y, Tsuneyama K, Oda S, Yokoi T. Kupffer cell-mediated exacerbation of methimazole-induced acute liver injury in rats. J Appl Toxicol, 2016; 36: 702-715.
- Takai S, Oda S, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Establishment of a mouse model for amiodarone-induced liver injury and analyses of its hepatotoxic mechanism. J Appl Toxicol, 2016; 36: 35-47.
- 織田進吾, 横井 毅. いまさら聞けない薬物動態Q&A: 薬剤師が知っておきたい抱合酵素とその基質について教えてください. 月刊薬事, 2016; 58: 685-690.
- 2015
- Iwamura A, Ito M, Mitsui H, Hasegawa J, Kosaka K, Kino I, Tsuda M, Nakajima M, Yokoi T, Kume T. Toxicological evaluation of acyl glucuronides utilizing half-lives, peptide adducts, and immunostimulation assays. Toxicol In Vitro, 2015; 30: 241-249.
- Nakajima A, Aoyama Y, Shin EJ, Nam Y, Kim HC, Nagai T, Yokosuka A, Mimaki Y, Yokoi T, Ohizumi Y, Yamada K. Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Abeta levels in a triple transgenic mouse model of Alzheimer's disease (3XTg-AD). Behav Brain Res, 2015; 289: 69-77.
- Tomida T, Okamura H, Satsukawa M, Yokoi T, Konno Y. Multiparametric assay using HepaRG cells for predicting drug-induced liver injury. Toxicol Lett, 2015; 236: 16-24.
- Iida A, Sasaki E, Yano A, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Carbamazepine-induced liver injury requires CYP3A-mediated metabolism and glutathione depletion in rats. Drug Metab Dispos, 2015; 43: 958-968.
- Oda S, Fujiwara R, Kutsuno Y, Fukami T, Itoh T, Yokoi T, Nakajima M. Targeted screen for human UDP-glucuronosyltransferases inhibitors and the evaluation of potential drug-drug interactions with zafirlukast. Drug Metab Dispos, 2015; 43: 812-818.
- Nakano M, Fukushima Y, Yokota S, Fukami T, Takamiya M, Aoki Y, Yokoi T, Nakajima M. CYP2A7 pseudogene transcript affects CYP2A6 expression in human liver by acting as a decoy for miR-126. Drug Metab Dispos, 2015; 43: 703-712.
- Lim YP, Cheng CH, Chen WC, Chang SY, Hung DZ, Chen JJ, Wan L, Ma WC, Lin YH, Chen CY, Yokoi T, Nakajima M, Chen CJ. Allyl isothiocyanate (AITC) inhibits pregnane X receptor (PXR) and constitutive androstane receptor (CAR) activation and protects against acetaminophen- and amiodarone-induced cytotoxicity. Arch Toxicol, 2015; 89: 57-72.
- Takai S, Higuchi S, Yano A, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Involvement of immune- and inflammatory-related factors in flucloxacillin-induced liver injury in mice. J Appl Toxicol, 2015; 35: 142-151.
- Oda S, Yokoi T. Establishment of animal models of drug-induced liver injury and analysis of possible mechanisms. Yakugaku Zasshi, 2015; 135: 579-588.
- Oda S, Fukami T, Yokoi T, Nakajima M. A comprehensive review of UDP-glucuronosyltransferase and esterases for drug development. Drug Metab Pharmacokinet, 2015; 30: 30-51.
- 2014
- Ito Y, Fukami T, Yokoi T, Nakajima M. An orphan esterase ABHD10 modulates probenecid acyl glucuronidation in human liver. Drug Metab Dispos, 2014; 42: 2109-2116.
- Sasaki E, Iwamura A, Tsuneyama K, Fukami T, Nakajima M, Kume T, Yokoi T. Role of cytochrome P450-mediated metabolism and identification of novel thiol-conjugated metabolites in mice with phenytoin-induced liver injury. Toxicol Lett, 2014; 232: 79-88.
- Lim YP, Chen WC, Cheng CH, Ma WC, Lin YH, Chen CY, Hung DZ, Chen JJ, Yokoi T, Nakajima M, Chen CJ. Inhibition of cytochrome P450 2C9 expression and activity in vitro by allyl isothiocyanate. Planta Med, 2014; 80: 1097-1106.
- Kurth MJ, Yokoi T, Gershwin ME. Halothane-induced hepatitis: paradigm or paradox for drug-induced liver injury. Hepatology, 2014; 60: 1473-1475.
- Yamaura Y, Nakajima M, Tatsumi N, Takagi S, Fukami T, Tsuneyama K, Yokoi T. Changes in the expression of miRNAs at the pericentral and periportal regions of the rat liver in response to hepatocellular injury: comparison with the changes in the expression of plasma miRNAs. Toxicology, 2014; 322: 89-98.
- Oda Y, Nakajima M, Tsuneyama K, Takamiya M, Aoki Y, Fukami T, Yokoi T. Retinoid X receptor alpha in human liver is regulated by miR-34a. Biochem Pharmacol, 2014; 90: 179-187.
- Shimizu M, Fukami T, Nakajima M, Yokoi T. Screening of specific inhibitors for human carboxylesterases or arylacetamide deacetylase. Drug Metab Dispos, 2014; 42: 1103-1109.
- Yano A, Oda S, Fukami T, Nakajima M, Yokoi T. Development of a cell-based assay system considering drug metabolism and immune- and inflammatory-related factors for the risk assessment of drug-induced liver injury. Toxicol Lett, 2014; 228: 13-24.
- Tang SC, Sparidans RW, Cheung KL, Fukami T, Durmus S, Wagenaar E, Yokoi T, van Vlijmen BJ, Beijnen JH, Schinkel AH. P-glycoprotein, CYP3A, and plasma carboxylesterase determine brain and blood disposition of the mTOR Inhibitor everolimus (Afinitor) in mice. Clin Cancer Res, 2014; 20: 3133-3145.
- Endo S, Yano A, Fukami T, Nakajima M, Yokoi T. Involvement of miRNAs in the early phase of halothane-induced liver injury. Toxicology, 2014; 319: 75-84.
- Takahashi K, Tatsumi N, Fukami T, Yokoi T, Nakajima M. Integrated analysis of rifampicin-induced microRNA and gene expression changes in human hepatocytes. Drug Metab Pharmacokinet, 2014; 29: 333-340.
- Shimizu M, Fukami T, Ito Y, Kurokawa T, Kariya M, Nakajima M, Yokoi T. Indiplon is hydrolyzed by arylacetamide deacetylase in human liver. Drug Metab Dispos, 2014; 42: 751-758.
- Oda S, Fukami T, Yokoi T, Nakajima M. Epigenetic regulation of the tissue-specific expression of human UDP-glucuronosyltransferase (UGT) 1A10. Biochem Pharmacol, 2014; 87: 660-667.
- Kuno S, Sakurai F, Shimizu K, Matsumura N, Kim S, Watanabe H, Tashiro K, Tachibana M, Yokoi T, Mizuguchi H. Development of mice exhibiting hepatic microsomal activity of human CYP3A4 comparable to that in human liver microsomes by intravenous administration of an adenovirus vector expressing human CYP3A4. Drug Metab Pharmacokinet, 2014; 29: 296-304.
- Matsuo K, Sasaki E, Higuchi S, Takai S, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Involvement of oxidative stress and immune- and inflammation-related factors in azathioprine-induced liver injury. Toxicol Lett, 2014; 224: 215-224.
- Muta K, Fukami T, Nakajima M, Yokoi T. N-Glycosylation during translation is essential for human arylacetamide deacetylase enzyme activity. Biochem Pharmacol, 2014; 87: 352-359.
- Miyashita T, Kimura K, Fukami T, Nakajima M, Yokoi T. Evaluation and mechanistic analysis of the cytotoxicity of the acyl glucuronide of nonsteroidal anti-inflammatory drugs. Drug Metab Dispos, 2014; 42: 1-8.
- Takahashi K, Oda Y, Toyoda Y, Fukami T, Yokoi T, Nakajima M. Regulation of cytochrome b5 expression by miR-223 in human liver: effects on cytochrome P450 activities. Pharm Res, 2014; 31: 780-794.
- 織田進吾, 横井 毅. 薬物性肝障害における免疫・炎症因子の関与. 日本皮膚アレルギー・接触皮膚炎学会雑誌, 2014; 8: 239-248.
- 横井 毅.肝障害とバイオマーカーとしてのmiRNA. 谷本学校 毒性質問箱, 2014; 16:56-67.
- Yokoi T. New prospectives and understanding in drug-induced liver injury considering drug metabolism and immune- and inflammation-related factors. Nihon Yakurigaku Zasshi, 2014; 144: 22-27.
- 横井 毅.薬物代謝と肝障害. 月刊薬事, 2014 56: 21-25.
- Miki Nakajima and Tsuyoshi Yokoi. MicroRNA-regulation of P450 and pharmacogenetics. Sandosh Padmanabhan Ed. Handbook Pharmacogenomics and Stratified Medicines, Elsevier, Watham, pp.385-401, 2014.
- 2013
- Sasaki E, Matsuo K, Iida A, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. A novel mouse model for phenytoin-induced liver injury: involvement of immune-related factors and P450-mediated metabolism. Toxicol Sci, 2013; 136: 250-263.
- Takahashi K, Yokota S, Tatsumi N, Fukami T, Yokoi T, Nakajima M. Cigarette smoking substantially alters plasma microRNA profiles in healthy subjects. Toxicol Appl Pharmacol, 2013; 272: 154-160.
- Kato Y, Izukawa T, Oda S, Fukami T, Finel M, Yokoi T, Nakajima M. Human UDP-glucuronosyltransferase (UGT) 2B10 in drug N-glucuronidation: substrate screening and comparison with UGT1A3 and UGT1A4. Drug Metab Dispos, 2013; 41: 1389-1397.
- Higuchi R, Fukami T, Nakajima M, Yokoi T. Prilocaine- and lidocaine-induced methemoglobinemia is caused by human carboxylesterase-, CYP2E1-, and CYP3A4-mediated metabolic activation. Drug Metab Dispos, 2013; 41: 1220-1230.
- Oda S, Fukami T, Yokoi T, Nakajima M. Epigenetic regulation is a crucial factor in the repression of UGT1A1 expression in the human kidney. Drug Metab Dispos, 2013; 41: 1738-1743.
- Poon CH, Wong TY, Wang Y, Tsuchiya Y, Nakajima M, Yokoi T, Leung LK. The citrus flavanone naringenin suppresses CYP1B1 transactivation through antagonising xenobiotic-responsive element binding. Br J Nutr, 2013; 109: 1598-1605.
- Kataoka M, Terashima Y, Mizuno K, Masaoka Y, Sakuma S, Yokoi T, Yamashita S. Establishment of MDCKII cell monolayer with metabolic activity by CYP3A4 transduced with recombinant adenovirus. Drug Metab Pharmacokinet, 2013; 28: 125-131.
- Yokoi T. A new era in the study of individual differences in drug metabolism and pharmacokinetics. Drug Metab Pharmacokinet, 2013; 28: 1-2.
- Yokoi T, Nakajima M. microRNAs as mediators of drug toxicity. Annu Rev Pharmacol Toxicol, 2013; 53: 377-400.
- 横井 毅.体内動態と薬物相互作用の基礎と応用-食品成分への展開-.食品加工技術, 2013; 33: 7-13.
- 横井 毅. 薬物代謝反応・代謝酵素の多様性と薬物相互作用の予測 p198-204. 「In vitro毒性・動態評価の最前線」小島肇監修、シーエムシー出, 2013.
- 2012
- Kobayashi Y, Fukami T, Higuchi R, Nakajima M, Yokoi T. Metabolic activation by human arylacetamide deacetylase, CYP2E1, and CYP1A2 causes phenacetin-induced methemoglobinemia. Biochem Pharmacol, 2012; 84: 1196-1206.
- Kakuni M, Morita M, Matsuo K, Katoh Y, Nakajima M, Tateno C, Yokoi T. Chimeric mice with a humanized liver as an animal model of troglitazone-induced liver injury. Toxicol Lett, 2012; 214: 9-18.
- Higuchi S, Yano A, Takai S, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Metabolic activation and inflammation reactions involved in carbamazepine-induced liver injury. Toxicol Sci, 2012; 130: 4-16.
- Miyashita T, Toyoda Y, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Hepatoprotective effect of tamoxifen on steatosis and non-alcoholic steatohepatitis in mouse models. J Toxicol Sci, 2012; 37: 931-942.
- Yoshikawa Y, Miyashita T, Higuchi S, Tsuneyama K, Endo S, Tsukui T, Toyoda Y, Fukami T, Nakajima M, Yokoi T. Mechanisms of the hepatoprotective effects of tamoxifen against drug-induced and chemical-induced acute liver injuries. Toxicol Appl Pharmacol, 2012; 264: 42-50.
- Oda S, Nakajima M, Hatakeyama M, Fukami T, Yokoi T. Preparation of a specific monoclonal antibody against human UDP-glucuronosyltransferase (UGT) 1A9 and evaluation of UGT1A9 protein levels in human tissues. Drug Metab Dispos, 2012; 40: 1620-1627.
- Endo S, Toyoda Y, Fukami T, Nakajima M, Yokoi T. Stimulation of human monocytic THP-1 cells by metabolic activation of hepatotoxic drugs. Drug Metab Pharmacokinet, 2012; 27: 621-630.
- Kobayashi Y, Fukami T, Shimizu M, Nakajima M, Yokoi T. Contributions of arylacetamide deacetylase and carboxylesterase 2 to flutamide hydrolysis in human liver. Drug Metab Dispos, 2012; 40: 1080-1084.
- Shimizu M, Fukami T, Kobayashi Y, Takamiya M, Aoki Y, Nakajima M, Yokoi T. A novel polymorphic allele of human arylacetamide deacetylase leads to decreased enzyme activity. Drug Metab Dispos, 2012; 40: 1183-1190.
- Kulthong K, Maniratanachote R, Kobayashi Y, Fukami T, Yokoi T. Effects of silver nanoparticles on rat hepatic cytochrome P450 enzyme activity. Xenobiotica, 2012; 42: 854-862.
- Oda Y, Nakajima M, Mohri T, Takamiya M, Aoki Y, Fukami T, Yokoi T. Aryl hydrocarbon receptor nuclear translocator in human liver is regulated by miR-24. Toxicol Appl Pharmacol, 2012; 260: 222-231.
- Iwamura A, Fukami T, Higuchi R, Nakajima M, Yokoi T. Human alpha/beta hydrolase domain containing 10 (ABHD10) is responsible enzyme for deglucuronidation of mycophenolic acid acyl-glucuronide in liver. J Biol Chem, 2012; 287: 9240-9249.
- Yano A, Higuchi S, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Involvement of immune-related factors in diclofenac-induced acute liver injury in mice. Toxicology, 2012; 293: 107-114.
- Taesotikul T, Nakajima M, Tassaneeyakul W, Yokoi T. Effects of Phyllanthus amarus on the pharmacokinetics of midazolam and cytochrome P450 activities in rats. Xenobiotica, 2012; 42: 641-648.
- Kobayashi M, Higuchi S, Ide M, Nishikawa S, Fukami T, Nakajima M, Yokoi T. Th2 cytokine-mediated methimazole-induced acute liver injury in mice. J Appl Toxicol, 2012; 32: 823-833.
- Kobayashi Y, Fukami T, Nakajima A, Watanabe A, Nakajima M, Yokoi T. Species differences in tissue distribution and enzyme activities of arylacetamide deacetylase in human, rat, and mouse. Drug Metab Dispos, 2012; 40: 671-679.
- Yamaura Y, Nakajima M, Takagi S, Fukami T, Tsuneyama K, Yokoi T. Plasma microRNA profiles in rat models of hepatocellular injury, cholestasis, and steatosis. PLoS One, 2012; 7: e30250.
- Toyoda Y, Endo S, Tsuneyama K, Miyashita T, Yano A, Fukami T, Nakajima M, Yokoi T. Mechanism of exacerbative effect of progesterone on drug-induced liver injury. Toxicol Sci, 2012; 126: 16-27.
- Kato Y, Nakajima M, Oda S, Fukami T, Yokoi T. Human UDP-glucuronosyltransferase isoforms involved in haloperidol glucuronidation and quantitative estimation of their contribution. Drug Metab Dispos, 2012; 40: 240-248.
- Higuchi S, Kobayashi M, Yano A, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Involvement of Th2 cytokines in the mouse model of flutamide-induced acute liver injury. J Appl Toxicol, 2012; 32: 815-822.
- Fukami T, Yokoi T. The emerging role of human esterases. Drug Metab Pharmacokinet, 2012; 27: 466-477.
- 2011
- Oda S, Nakajima M, Toyoda Y, Fukami T, Yokoi T. Progesterone receptor membrane component 1 modulates human cytochrome p450 activities in an isoform-dependent manner. Drug Metab Dispos, 2011; 39: 2057-2065.
- Nakajima A, Fukami T, Kobayashi Y, Watanabe A, Nakajima M, Yokoi T. Human arylacetamide deacetylase is responsible for deacetylation of rifamycins: rifampicin, rifabutin, and rifapentine. Biochem Pharmacol, 2011; 82: 1747-1756.
- Kida K, Nakajima M, Mohri T, Oda Y, Takagi S, Fukami T, Yokoi T. PPARalpha is regulated by miR-21 and miR-27b in human liver. Pharm Res, 2011; 28: 2467-2476.
- Hosomi H, Fukami T, Iwamura A, Nakajima M, Yokoi T. Development of a highly sensitive cytotoxicity assay system for CYP3A4-mediated metabolic activation. Drug Metab Dispos, 2011; 39: 1388-1395.
- Hioki T, Fukami T, Nakajima M, Yokoi T. Human paraoxonase 1 is the enzyme responsible for pilocarpine hydrolysis. Drug Metab Dispos, 2011; 39: 1345-1352.
- Usui T, Hashizume T, Katsumata T, Yokoi T, Komuro S. In vitro investigation of the glutathione transferase M1 and T1 null genotypes as risk factors for troglitazone-induced liver injury. Drug Metab Dispos, 2011; 39: 1303-1310.
- Toyoda Y, Miyashita T, Endo S, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Estradiol and progesterone modulate halothane-induced liver injury in mice. Toxicol Lett, 2011; 204: 17-24.
- Iwamura A, Fukami T, Hosomi H, Nakajima M, Yokoi T. CYP2C9-mediated metabolic activation of losartan detected by a highly sensitive cell-based screening assay. Drug Metab Dispos, 2011; 39: 838-846.
- Abe Y, Fujiwara R, Oda S, Yokoi T, Nakajima M. Interpretation of the effects of protein kinase C inhibitors on human UDP-glucuronosyltransferase 1A (UGT1A) proteins in cellulo. Drug Metab Pharmacokinet, 2011; 26: 256-265.
- Higuchi S, Kobayashi M, Yoshikawa Y, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. IL-4 mediates dicloxacillin-induced liver injury in mice. Toxicol Lett, 2011; 200: 139-145.
- Koga T, Fujiwara R, Nakajima M, Yokoi T. Toxicological evaluation of acyl glucuronides of nonsteroidal anti-inflammatory drugs using human embryonic kidney 293 cells stably expressing human UDP-glucuronosyltransferase and human hepatocytes. Drug Metab Dispos, 2011; 39: 54-60.
- Yokota S, Higashi E, Fukami T, Yokoi T, Nakajima M. Human CYP2A6 is regulated by nuclear factor-erythroid 2 related factor 2. Biochem Pharmacol, 2011; 81: 289-294.
- Mizuno K, Toyoda Y, Fukami T, Nakajima M, Yokoi T. Stimulation of pro-inflammatory responses by mebendazole in human monocytic THP-1 cells through an ERK signaling pathway. Arch Toxicol, 2011; 85: 199-207.
- Yokoi T, Nakajima M. Toxicological implications of modulation of gene expression by microRNAs. Toxicol Sci, 2011; 123: 1-14.
- Yokoi T. Current topics in drug metabolism and drug toxicity. Preface. Drug Metab Pharmacokinet, 2011; 26: 1-2.
- Nakajima M, Yokoi T. MicroRNAs from biology to future pharmacotherapy: regulation of cytochrome P450s and nuclear receptors. Pharmacol Ther, 2011; 131: 330-337.
- 横井 毅. 第II相代謝の評価と創薬 p224-231.「医薬品開発に必要なストラテジーと各種試験法」日本薬理学会編, 2011.
- 2010
- Watanabe A, Fukami T, Takahashi S, Kobayashi Y, Nakagawa N, Nakajima M, Yokoi T. Arylacetamide deacetylase is a determinant enzyme for the difference in hydrolase activities of phenacetin and acetaminophen. Drug Metab Dispos, 2010; 38: 1532-1537.
- Kobayashi M, Higuchi S, Mizuno K, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Interleukin-17 is involved in alpha-naphthylisothiocyanate-induced liver injury in mice. Toxicology, 2010; 275: 50-57.
- Fukami T, Nakajima M, Matsumoto I, Zen Y, Oda M, Yokoi T. Immunohistochemical analysis of CYP2A13 in various types of human lung cancers. Cancer Sci, 2010; 101: 1024-1028.
- Fukami T, Takahashi S, Nakagawa N, Maruichi T, Nakajima M, Yokoi T. In vitro evaluation of inhibitory effects of antidiabetic and antihyperlipidemic drugs on human carboxylesterase activities. Drug Metab Dispos, 2010; 38: 2173-2178.
- Mizuno K, Fukami T, Toyoda Y, Nakajima M, Yokoi T. Terbinafine stimulates the pro-inflammatory responses in human monocytic THP-1 cells through an ERK signaling pathway. Life Sci, 2010; 87: 537-544.
- Holmes RS, Wright MW, Laulederkind SJ, Cox LA, Hosokawa M, Imai T, Ishibashi S, Lehner R, Miyazaki M, Perkins EJ, Potter PM, Redinbo MR, Robert J, Satoh T, Yamashita T, Yan B, Yokoi T, Zechner R, Maltais LJ. Recommended nomenclature for five mammalian carboxylesterase gene families: human, mouse, and rat genes and proteins. Mamm Genome, 2010; 21: 427-441.
- Takagi S, Nakajima M, Kida K, Yamaura Y, Fukami T, Yokoi T. MicroRNAs regulate human hepatocyte nuclear factor 4alpha, modulating the expression of metabolic enzymes and cell cycle. J Biol Chem, 2010; 285: 4415-4422.
- Hosomi H, Akai S, Minami K, Yoshikawa Y, Fukami T, Nakajima M, Yokoi T. An in vitro drug-induced hepatotoxicity screening system using CYP3A4-expressing and gamma-glutamylcysteine synthetase knockdown cells. Toxicol In Vitro, 2010; 24: 1032-1038.
- Nakajima M, Koga T, Sakai H, Yamanaka H, Fujiwara R, Yokoi T. N-Glycosylation plays a role in protein folding of human UGT1A9. Biochem Pharmacol, 2010; 79: 1165-1172.
- Mohri T, Nakajima M, Fukami T, Takamiya M, Aoki Y, Yokoi T. Human CYP2E1 is regulated by miR-378. Biochem Pharmacol, 2010; 79: 1045-1052.
- Maruichi T, Fukami T, Nakajima M, Yokoi T. Transcriptional regulation of human carboxylesterase 1A1 by nuclear factor-erythroid 2 related factor 2 (Nrf2). Biochem Pharmacol, 2010; 79: 288-295.
- Fujiwara R, Nakajima M, Oda S, Yamanaka H, Ikushiro S, Sakaki T, Yokoi T. Interactions between human UDP-glucuronosyltransferase (UGT) 2B7 and UGT1A enzymes. J Pharm Sci, 2010; 99: 442-454.
- 横井 毅. 薬物動態関連遺伝子の多型と薬物相互作用. 臨床検査, 2010; 54: 1107-1113.
- 横井 毅. 薬物代謝異常と小胞体 p235-250. 「生物薬科学実験講座5、細胞の構造とオルガネラ」大熊勝治、中西義信編集、廣川書店, 2010.
- 横井 毅. 薬物代謝に関与する酵素とその反応機構 p43-68、 薬物代謝と毒性発現 p182-192.「薬物代謝学 医療薬学・医薬品開発の基礎として第3版」加藤隆一、山添 康、横井 毅編、 東京化学同人, 2010.
- Tsuyoshi Yokoi. Troglitazone p419-435. 「Adverse Drug Reactions」 Jack Uetrecht Ed. in Handbook of Expreimental Pharmacology 196, Springer-Verlag, Berlin, 2010.
- 2009
- Nakagawa N, Katoh M, Yoshioka Y, Nakajima M, Yokoi T. Inhibitory effects of Kampo medicine on human UGT2B7 activity. Drug Metab Pharmacokinet, 2009; 24: 490-499.
- Yoshikawa Y, Morita M, Hosomi H, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Knockdown of superoxide dismutase 2 enhances acetaminophen-induced hepatotoxicity in rat. Toxicology, 2009; 264: 89-95.
- Kobayashi E, Kobayashi M, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Halothane-induced liver injury is mediated by interleukin-17 in mice. Toxicol Sci, 2009; 111: 302-310.
- Komagata S, Nakajima M, Takagi S, Mohri T, Taniya T, Yokoi T. Human CYP24 catalyzing the inactivation of calcitriol is post-transcriptionally regulated by miR-125b. Mol Pharmacol, 2009; 76: 702-709.
- Yoshikawa Y, Hosomi H, Fukami T, Nakajima M, Yokoi T. Establishment of knockdown of superoxide dismutase 2 and expression of CYP3A4 cell system to evaluate drug-induced cytotoxicity. Toxicol In Vitro, 2009; 23: 1179-1187.
- Morita M, Akai S, Hosomi H, Tsuneyama K, Nakajima M, Yokoi T. Drug-induced hepatotoxicity test using gamma-glutamylcysteine synthetase knockdown rat. Toxicol Lett, 2009; 189: 159-165.
- Izukawa T, Nakajima M, Fujiwara R, Yamanaka H, Fukami T, Takamiya M, Aoki Y, Ikushiro S, Sakaki T, Yokoi T. Quantitative analysis of UDP-glucuronosyltransferase (UGT) 1A and UGT2B expression levels in human livers. Drug Metab Dispos, 2009; 37: 1759-1768.
- Watanabe A, Fukami T, Nakajima M, Takamiya M, Aoki Y, Yokoi T. Human arylacetamide deacetylase is a principal enzyme in flutamide hydrolysis. Drug Metab Dispos, 2009; 37: 1513-1520.
- Mohri T, Nakajima M, Takagi S, Komagata S, Yokoi T. MicroRNA regulates human vitamin D receptor. Int J Cancer, 2009; 125: 1328-1333.
- Volotinen M, Maenpaa J, Kankuri E, Oksala O, Pelkonen O, Nakajima M, Yokoi T, Hakkola J. Expression of cytochrome P450 (CYP) enzymes in human nonpigmented ciliary epithelial cells: induction of CYP1B1 expression by TCDD. Invest Ophthalmol Vis Sci, 2009; 50: 3099-3105.
- Takahashi S, Katoh M, Saitoh T, Nakajima M, Yokoi T. Different inhibitory effects in rat and human carboxylesterases. Drug Metab Dispos, 2009; 37: 956-961.
- Ishihara K, Katsutani N, Asai N, Inomata A, Uemura Y, Suganuma A, Sawada K, Yokoi T, Aoki T. Identification of urinary biomarkers useful for distinguishing a difference in mechanism of toxicity in rat model of cholestasis. Basic Clin Pharmacol Toxicol, 2009; 105: 156-166.
- Fujiwara R, Nakajima M, Yamamoto T, Nagao H, Yokoi T. In silico and in vitro approaches to elucidate the thermal stability of human UDP-glucuronosyltransferase (UGT) 1A9. Drug Metab Pharmacokinet, 2009; 24: 235-244.
- Katoh M, Yoshioka Y, Nakagawa N, Yokoi T. Effects of Japanese herbal medicine, Kampo, on human UGT1A1 activity. Drug Metab Pharmacokinet, 2009; 24: 226-234.
- Mizuno K, Katoh M, Okumura H, Nakagawa N, Negishi T, Hashizume T, Nakajima M, Yokoi T. Metabolic activation of benzodiazepines by CYP3A4. Drug Metab Dispos, 2009; 37: 345-351.
- Fujiwara R, Nakajima M, Yamanaka H, Yokoi T. Key amino acid residues responsible for the differences in substrate specificity of human UDP-glucuronosyltransferase (UGT)1A9 and UGT1A8. Drug Metab Dispos, 2009; 37: 41-46.
- Yokoi T. Essentials for starting a pediatric clinical study (1): Pharmacokinetics in children. J Toxicol Sci, 2009; 34 Suppl 2: SP307-312.
- 横井 毅. 薬物動態と医薬品の薬効・副作用:代謝を中心として.治療学, 2009; 43: 1262-1266.
- 横井 毅. 第II相代謝の評価と創薬:化合物から医薬品にするために必要な薬物動態試験. 日本薬理学雑誌, 2009; 134: 334-337.
- 横井 毅. 薬物動態関連遺伝子の多型と薬物相互作用.DNA多型, 2009; 17: 6-10.
- 横井 毅. 薬物の作用, 薬物動態の基本 p9-20.「医薬品トキシコロジー 改訂第4版」佐藤哲男、仮家公夫、北田光一編、南江堂, 2009.
- 横井 毅. 薬物療法の個人差と薬物代謝酵素の遺伝子多型 p43-47. ひらかれた小児リウマチ治療、セカンド出版, 2009.
- 横井 毅.毒科学とゲノムサイエンス・トキシコゲノミクス p56-60「標準医療薬学 薬理学」辻本豪三、小池勝夫編、医学書院, 2009.
Research Keywords
adverse drug reactions, drug metabolism and pharmacokinetics, ADME-Tox, immunotoxicology, toxicology, drug safety, histopathology drug-induced liver injury, drug-induced-kidney-injury animal model, biomarker, immunostaining, HPLC, FACS reactive metabolite, oxidative stress, non-coding RNA, microRNA
| Program | Integrated Medicine |
|---|---|
| Field | Clinical Pharmacology |
| Department | Toxicogenomics |