Laboratories
- Back
- Top > Laboratories > Clinical Pharmacology > Neuropsychopharmacology and Hospital Pharamacy (Cooperating field)
Clinical PharmacologyNeuropsychopharmacology and Hospital Pharamacy (Cooperating field)
Introduction
The brain controls all body activities, ranging from heart rate to memory. Each brain function is operated by particular neural circuits making synaptic connection. One of most important feature of synapses is the activity-dependent plasticity. The long-term objective in our laboratory is twofold. One is to define the molecular and cellular mechanisms that regulate synaptic plasticity associated with learning and memory. In particular, we are interested in molecular and cellular mechanisms that are responsible for working/short-term memory and reference/long-term memory. The other is to define the pathogenesis/pathophysiology of neuropsychiatric disorders, including schizophrenia, drug addiction and Alzheimer’s disease.
Research Projects
1. Research projects for mental disorders
i) Research project for schizophrenia
Both genetic and environmental factors play a role in the pathology of schizophrenia. Recently, genetic susceptibility factors for the disorder have become available, which include neuregulin-1, dysbindin, and disrupted-in-schizophrenia 1 (DISC1). Epidemiological studies have identified environmental factors for schizophrenia, and maternal viral infection during pregnancy is regarded as the most promising one. Viral infection in the second trimester of pregnancy in humans increases the risk of subsequently developing schizophrenia in adolescence/adulthood. A possible interaction between environmental and genetic susceptibility factors, especially during neurodevelopment, is proposed for the disease etiology.
We focus on phenotypic analyses at behavioral, neurochemical and neuroanatomical levels in mice with mutant schizophrenia susceptibility genes such as DISC1. We also study a possible interaction of genetic and environmental factors in schizophrenia by using a genetically-engineered mouse models under exposure to the synthetic double strand RNA polyriboinosinic-polyribocytidilic acid [polyI:C, a toll-like receptor 3 (TLR3) ligand that induces strong innate immune response]. PolyI:C has been used to mimic a viral infection during neurodevelopment for modeling schizophrenia in mice (Fig. 1).
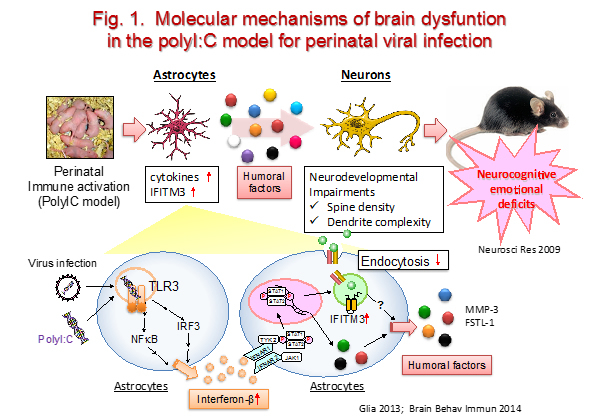
ii) Research project for stress-related disorders
Stress plays a significant role in the development of various neuropsychiatric disorders, e.g., anxiety disorder, major depressive disorder, and bipolar disorder. Previously, we demonstrated that chronic social isolation or restraint stress impaired memory and emotional behavior, which was accompanied by a reduction of the mRNA levels of a brain-specific transcription factor, neuronal Per Arnt Sim domain 4 (Npas4), in the hippocampus. We also demonstrated that the transcription of Npas4 was downregulated by acute restraint stress via the binding of the agonist-bound glucocorticoid receptor (GR) to its promoter and DNA methylation of its promoter (Fig. 2, 3).
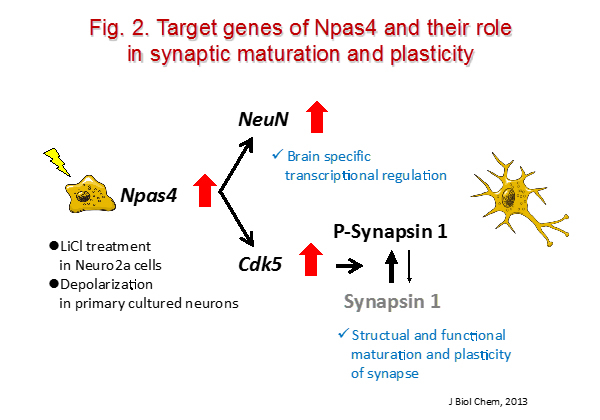
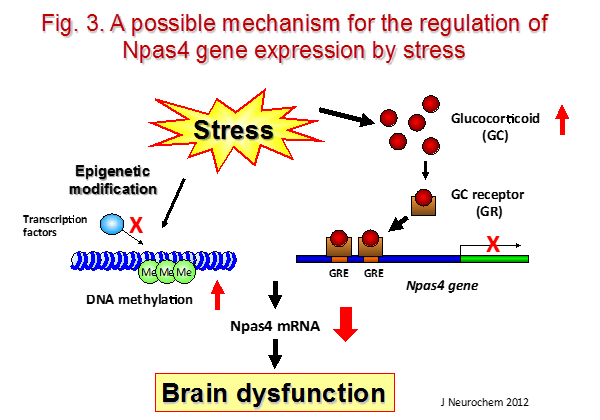
iii) Research project for drug addiction/dependence
Drug addiction/dependence is defined as a chronically relapsing disorder that is characterized by compulsive drug taking, inability to limit intake, and intense drug cravings. The positive reinforcing/rewarding effects of drugs primarily depend on the mesocorticolimbic dopamine system innervating the nucleus accumbens while the craving for drugs is associated with activation of the prefrontal cortex. The chronic intake of drugs causes homeostatic molecular and functional changes in synapses, which may be critically associated with the development of drug dependence. We have demonstrated that various cytokines such as TNF-α and GDNF, and proteinases such as tissue plasminogen activator (tPA) and matrix metalloproteinase (MMP-2/MMP-9) are produced in the brain on treatment with drugs of abuse, and play a role in the development of drug dependence. These endogenous modulators of drug dependence are classified into two groups, pro-addictive and anti-addictive factors. We have proposed that an imbalance between pro-addictive and anti-addictive factors contributes to the development and relapse of drug dependence. Targeting these endogenous modulators would provide new therapeutic approaches to the treatment of drug dependence (Fig. 4).
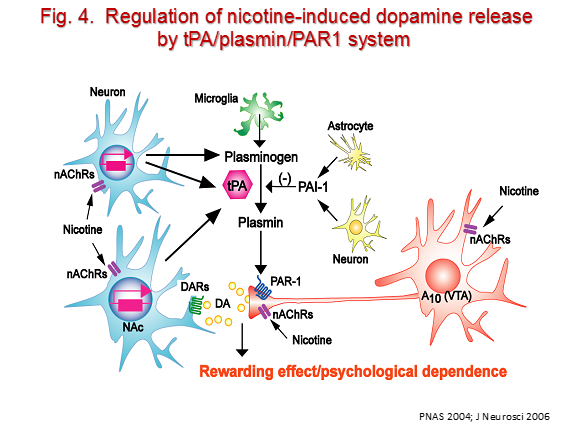
iv) Research project for decision-making
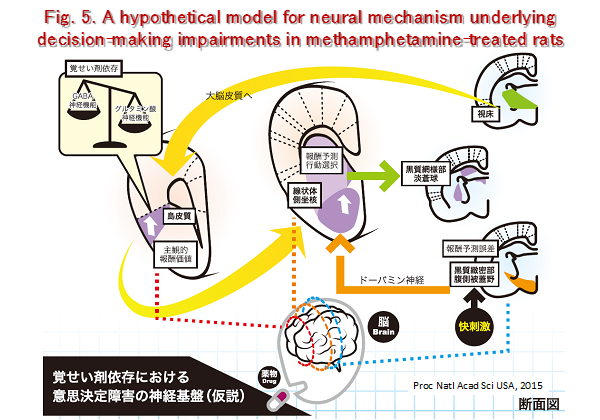
Decision-making is a key activity of everyday life. Consequently, disturbances in the ability to make appropriate decisions or anticipate their possible consequences can result in massive social, medical, and financial problems. Patients suffering from neuropsychiatric disorders such as substance-related and addictive disorders have impairments in decision-making, which may be associated with their behavioral abnormalities. However, the neuronal mechanisms underlying such impairments are largely unknown. Using an originally developed gambling test for rodents, we demonstrated that methamphetamine (METH)-dependent rats choose a high-risk/high-reward option more frequently, and assign higher subjective value to high returns, than control rats, suggesting that decision-making is impaired in the drug-dependent animals. We seek to clarify the underlying neural mechanisms for the impairment of decision-making in methamphetamine-dependent rats (Fig. 5).
2. Research projects for neurodegenerative disorders
i) Research project for Alzheimer’s disease
Alzheimer’s disease (AD) is a progressive neurodegenerative process characterized by senile plaques, neurofibrillary tangles and neuronal loss. A pathologic hallmark of AD is deposition of amyloid-β peptide (Aβ), a 39-43 amino acid peptide derived from the transmembrane amyloid precursor protein (APP). Fibrillar Aβ deposits are found in extracellular senile plaque cores and are associated with neurodegeneration in later stages of AD. We study the mechanisms underlying the Aβ-induced neurotoxicity in vivo, by directly injecting Aβ into the lateral ventricle. We have already discovered novel compounds to prevent the Aβ-induced neurotoxicity.
ii) Research project for neurodegenerative seizures
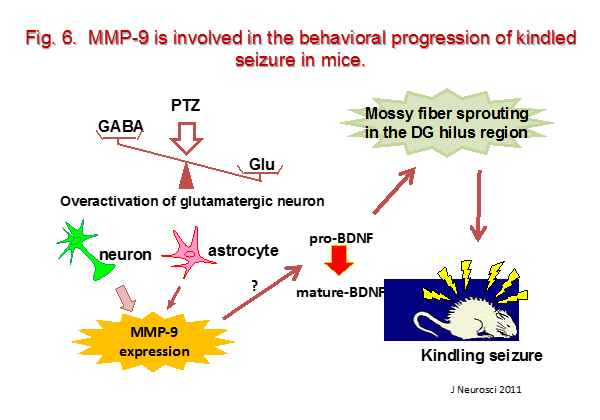
Seizures cause brain injury via a number of mechanisms, potentially contributing to neurologic and cognitive deficits in epilepsy patients. Although seizures induce neuronal death in some situations, they also can produce nonlethal pathophysiologic effects on neuronal structures and functions. Kindling is an experimental epilepsy model in which repeated electrical or chemical stimulation of certain forebrain structures triggers progressively more intense electroencephalographic and behavioral seizure activity. Once established, kindling results in a permanent state of seizure susceptibility, which may manifest as spontaneous epileptiform seizures. Kindling has recently been shown to induce a variety of permanent structural changes in the brain, including sprouting of the mossy fiber pathway that originates from hippocampal dentate gyrus granule cells and neuronal loss in the hippocampus. Using a pentylenetetrazole (PTZ)-induced kindling model in mice, we have been exploring the neuronal mechanism for seizure susceptibility leading to variety of permanent structural changes (Fig. 6).
3. Research projects for basic mechanisms of higher order brain functions
i) Research project for basic mechanisms of recognition memory
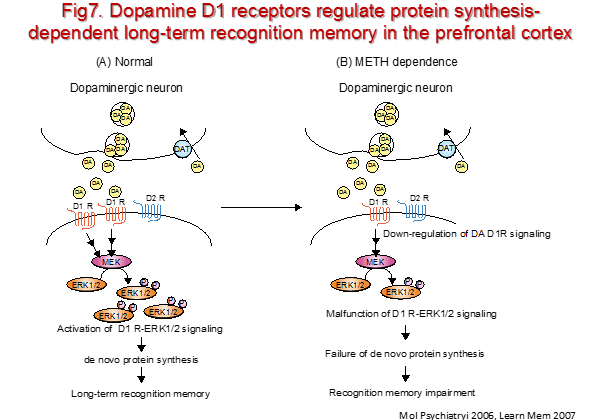
Recognition memory is a fundamental facet of ability to remember, and an integral component of the class of memory lost in amnesia. The ability to discriminate familiar from novel stimuli is supported by this form of memory and is widely used as an assay in animals. Recent works highlight a major role in recognition memory for the perirhinal cortex because lesions or transient inactivation of perirhinal cortex consistently disrupt performance on familiarity discrimination tasks with object in both primates and rats. We have previously demonstrated that the activation of ERK1/2 following stimulation of dopamine D1 receptors in the prefrontal cortex is necessary for the protein synthesis-dependent long-term retention of recognition memory (Fig. 7). Now, we focus on the possible interaction between dopamine and glutamate systems in protein synthesis-dependent long-term retention of recognition memory. Furthermore, we look for target genes of the dopamine D1 receptor-ERK1/2 signaling which are responsible for long-term recognition memory.
ii) Research project for basic mechanisms of synaptic plasticity
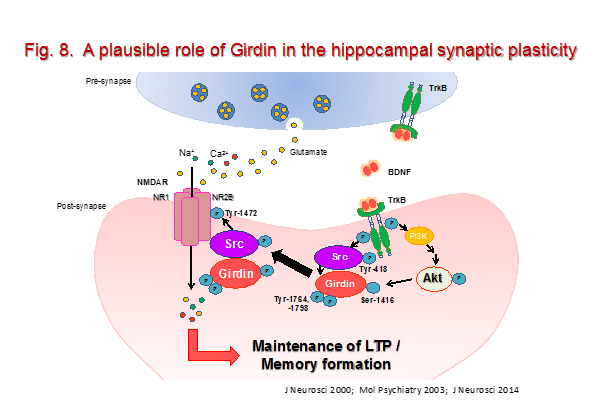
It has been widely believed that the synaptic plasticity in hippocampal neurons is related to the formation of various memories. Synaptic plasticity refers to the ability of synapses to vary their efficacy of chemical or electrical transmission over time in response to activities. Regulation of synaptic transmission and strength may be mediated by several events including changes in expression of synaptic surface receptors and neurotransmitters, altered neurite conductance, receptor subunit phosphorylation, postsynaptic calcium release and cytoskeletal remodeling. Neurotrophins are also considered powerful molecular mediators in synaptic plasticity. For example, brain-derived neurotrophic factor (BDNF) as well as its receptor TrkB have emerged as key molecules in the neurobiological mechanisms related to learning and memory. TrkB is known to activate Ras/extracellular signal-regulated kinase (ERK), phospholipase C (PLC) pathways and phosphatidylinositol 3-kinase (PI3-K)/Akt. Serine/threonine kinase Akt has been linked to brain development, ageing, and neurodegenerative and psychiatric disorders via the phosphorylation of a wide variety of its substrates. Girdin (also known as APE, GIV, HkRP1) has been identified as an actin-binding Akt substrate that has been reported to regulate migration of various cells. We have recently demonstrated that Girdin S1416 was phosphorylated in an activity-dependent manner, and this phosphorylation was essential for spine morphogenesis, maintenance of hippocampal LTP and memory formation. Furthermore, Girdin interacts with Src and NR2B subunit of NMDA receptors, leading to phosphorylation of the NR2B subunit and NMDA receptor activation. Our findings suggest that activity-dependent Girdin phosphorylation at S1416 induced by Akt is crucial for NMDA receptor activation associated with neuronal plasticity underlying hippocampal memory formation (Fig. 8).
iii) Research project for basic mechanisms of emotion
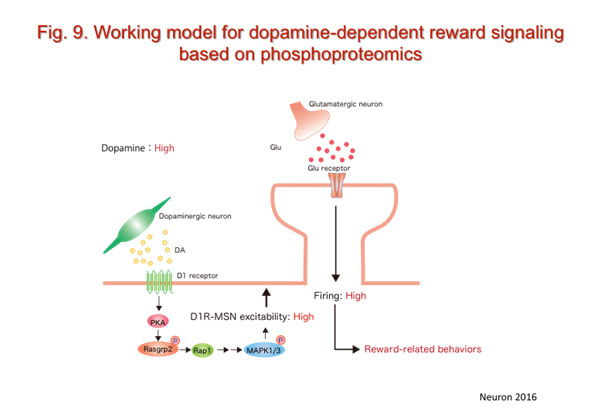
Collaboration with Prof. Kaibuchi (Nagoya Univ.), we recently developed a phospho-proteomic analysis method to identify known and novel PKA substrates downstream of the dopamine D1 receptors and obtained more than one hundred candidate substrates, including Rap1 GEF (Rasgrp2). We found a novel dopamine-PKA-Rap1-MAPK intracellular signaling mechanism in D1 receptor-expressing medium spiny neurons that increases neuronal excitability to enhance reward-related behaviors (Fig. 9).
Faculty Members
| Faculty | Position | Department |
|---|---|---|
| YAMADA Kiyofumi | Professor | Neuropsychopharmacology/Pharmacy |
| NAGAI Taku | Associate Professor | Neuropsychopharmacology/Pharmacy |
| ITOH Norimichi | Assistant Professor | Neuropsychopharmacology/Pharmacy |
| SAWAHATA Masahito | Assistant Professor | Neuropsychopharmacology |
Bibliography
- 2016
- Itoh N, Enomoto A, Nagai T, Takahashi M, Yamada K. Molecular mechanism linking BDNF/TrkB signaling with the NMDA receptor in memory: The role of Girdin in the CNS. Rev Neurosci., 2016; 27: 481-90.
- Aoyama Y, Toriumi K, Mouri A, Hattori T, Ueda E, Shimato A, Sakakibara N, Soh Y, Mamiya T, Nagai T, Kim HC, Hiramatsu M, Nabeshima T, Yamada K. Prenatal nicotine exposure impairs the proliferation of neuronal progenitors, leading to fewer glutamatergic neurons in the medial prefrontal cortex. Neuropsychopharmacology, 2016; 41: 578-589.
- Nagai T, Nakamuta S, Kuroda K, Nakauchi S, Nishioka T, Takano T, Zhang X, Tsuboi D, Funahashi Y, Nakano T, Yoshimoto J, Kobayashi K, Uchigashima M, Watanabe M, Miura M, Nishi A, Kobayashi K, Yamada K, Amano M, Kaibuchi K. Phospho-proteomics of the dopamine pathway enables discovery of Rap1 activation as a reward signal in vivo. Neuron, 2016; 89: 550-565.
- 2015
- Ibi D, Yamada K. Therapeutic targets for neurodevelopmental disorders emerging from animal models with perinatal immune activation. Int. J. Mol. Sci., 2015; 16: 1-12 (doi:10.3390/ijms161226092)
- Mizoguchi H, Katahira K, Inutsuka A, Fukumoto K, Wang T, Nagai T, Sato J, Sawada M, Ohira H, Yamanaka A, Yamada K. Insular neural system controls decision-making in healthy and methamphetamine-treated rats. Proc. Natl. Acad. Sci. USA., 2015; 112: E3930-3939.
- Furukawa-Hibi Y, Yun J, Nagai T, Yamada K. Stress increases DNA methylation of the neuronal PAS domain 4 (Npas4) gene. Neuroreport, 2015; 26: 827-832.
- Nakajima A, Aoyama Y, Shin, EJ, Nam Y, Kim HC, Nagai T, Yokosuka A, Mimaki Y, Yokoi, T, Ohizumi Y, Yamada K. Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Aβ levels in a triple transgenic mouse model of Alzheimer's disease (3XTg-AD). Behav. Brain Res., 2015; 289: 69-77.
- Yamada K, Nabeshima T. Pharmacist-managed clinics for patient education and counseling in Japan: current status and future perspectives. J. Pharm. Health Care Sci., 2015; 1: 2 (doi:10.1186/s40780-014-0001-4)
- Ichikawa K, van Ingen J, Koh WJ, Wagner D, Salfinger M, Inagaki T, Uchiya KI, Nakagawa T, Ogawa K, Yamada K, Yagi T. Genetic diversity of clinical Mycobacterium avium subsp. hominissuis and Mycobacterium intracellulare isolates causing pulmonary diseases recovered from different geographical regions. Infect Genet Evol., 2015; 36: 250-255.
- Yamamura K, Yano K, Kirooka Y, Hirashiki A, Murohara T, Yamada K. A successful case of a ptient undergoing warfarin and S-1 therapy using internet-based control of hemo-measured PT-INR. YAKUGAKU ZASSHI, 2015; 135: 925-927.
- Kato H, Miyazaki M, Takeuchi M, Tsukuura H, Sugishita M, Noda N, Yamada K. A retrospective study to identify risk factors for somnolence and dizziness in patients treated with pregabalin. J. Pharm. Health Care Sci., 2015; 1: 22 (10.1186/s40780-015-0022-7).
- 2014
- Nakai T, Nagai T, Tanaka M, Itoh N, Asai N, Enomoto A, Asai M, Yamada S, Saifullah MAB, Sokabe M, Takahashi M, and Yamada K. Girdin phosphorylation is crucial for synaptic plasticity and memory: a potential role in the interaction of BDNF/TrkB/Akt signaling with NMDA receptor. J. Neurosci., 2014; 34: 14995-15008.
- Nakajima A, Ibi D, Nagai T, Yamada S, Nabeshima T, Yamada K. Induction of interferone-induced transmembrane protein 3 gene expression by lipopolysaccharide in astrocytes. Eur. J. Pharmacol., 2014; 745: 166-175.
- Yamada S, Nagai T, Nakai T, Ibi D, Nakajima A, Yamada K: Matrix metalloproteinase-3 is a possible mediator of neurodevelopmental impairment due to polyI:C-induced innate immune activation of astrocytes. Brain Behav Immun., 2014; 38: 272-282.
- Nakai T, Nagai T, Wang R, Yamada S, Kuroda K, Kaibuchi K, Yamada K: Alterations of GABAergic and dopaminergic system in mutant mice with disruption of exons 2 and 3 of the Disc1 gene. Neurochem. Int., 2014; 74: 74-83.
- Nakajima N, Ohizumi Y, Yamada K. Anti-dementia activity of nobiletin, a citrus flavonoid. Clin. Psychopharmacol. Neurosci., 2014; 12: 75-82.
- 2013
- Mizoguchi H, Yamada K. Roles of matrix metalloproteinases and their targets in epileptogenesis and seizures. Clin. Psychopharmacol. Neurosci., 2013; 11:45-52.
- Mouri A, Nagai T, Ibi D, Yamada K. Animal models of schizophrenia for molecular and pharmacological intervention and potential candidate molecules. Neurobiol. Dis., 2013; 53: 61-74.
- Nakajima A, Aoyama Y, Nguyen TTL, Shin EJ, Kim HC, Yamada S, Nakai T, Nagai T, Yokosuka A, Mimaki Y, Ohizumi Y, Yamada K. Nobiletin, a citrus flavonoid, ameliorates cognitive impairment, oxidative burden, and hyperphosphorylation of tau in senescence-accelerated mouse. Behav. Brain Res., 2013; 250: 351-360.
- Miyazaki M, Noda Y, Mouri A, Kobayashi K, Mishina M, Nabeshima N, Yamada K. Role of convergent activation of glutamatergic and dopaminergic systems in the nucleus accumbens in the development of methamphetamine psychosis and dependence. Int. J. Neuropsychopharmacol., 2013; 16: 1341-1350.
- Yun J, Nagai T, Furukawa-Hibi Y, Kuroda K, Kaibuchi K, Yamada K. Neuronal Per Arnt Sim (PAS) domain protein 4 (Npas4) regulates neurite outgrowth and phosphorylation of synapsin I. J. Biol. Chem., 2013; 288: 2655-2664.
- Ibi D, Nagai T, Nakajima A, Mizoguchi H, Kawase T, Tsuboi D, Kano S, Sato Y, Hayakawa M, Lange UC, Adams DJ, Surani MA, Satoh T, Sawa A, Kaibuchi K, Nabeshima T, Yamada K. Astroglial IFITM3 mediates neuronal impairments following neonatal immune challenge in mice. Glia, 2013; 61: 679-693.
- 2012
- Furukawa-Hibi Y, Nitta A, Fukumitsu H, Somiya H, Toriumi K, Furukawa S, Nabeshima T, Yamada K. Absence of SHATI/Nat81 reduces social interaction in mice. Neurosci. Lett., 2012; 526: 79-84.
- Nagai T, Yu J, Kitahara Y, Nabeshima T, Yamada K. D-serine ameliorates neonatal polyI:C treatment-induced emotional and cognitive impairments in adult mice. J. Pharmacol. Sci., 2012; 120: 213-227.
- Furukawa-Hibi Y, Yun J, Nagai T, Yamada K. Transcriptional suppression of the neuronal PAS domain 4 (Npas4) gene by stress via the binding of agonist-bound glucocorticoid receptor to its promoter. J. Neurochem., 2012; 123: 866-875.
- Watanabe N, Yamamura K, Suzuki Y, Umegaki H, Shigeno K, Sai Y, Miyamoto K, Yamada K. Pharmacist-based donepezil outpatient consultation service to improve medication persistence. Patient Preference Adherence, 2012; 6: 1-7.
- Takuma K, Mizoguchi H, Funatsu Y, Kitahara Y, Ibi D, Kamei H, Matsuda T, Koike K, Inoue M, Nagai T, Yamada K. Placental extract improves hippocampal neuronal loss and fear memory impairment resulting from chronic restraint stress in ovariectomized mice. J. Pharmacol. Sci., 2012; 120: 89-97.
- Mizuno T, Sato W, Ishikawa K, Shinjo H, Miyagawa Y, Noda Y Imai E, Yamada K. KDIGO criteria could be useful outcome predictor of cisplatin-induced acute kidney injury. Oncology, 2012; 82: 354-359.
- Takuma K, Mizoguchi H, Funatsu Y, Hoshina Y, Himeno Y, Fukuzaki E, Kitahara Y, Arai S, Ibi D, Kamei H, Matsuda T, Koike K, Inoue M, Nagai T, Yamada K. Combination of chronic stress and ovariectomy causes conditioned fear memory deficits and hippocampal cholinergic neuronal loss in mice. Neuroscience, 2012; 207: 261-273.
- Mizuno T, Ito K, Miyagawa Y, Kimura K, Suzuki Y, Mizuno M, Ito Y, Funahasi Y, Hattori R, Gotoh M, Noda Y, Yamada K. Renal impairment after laparoscopic radical nephrectomy affects hypoglycaemic therapy. J Clinic. Pharm. Therapeu., 2012; 37: 49-52.
- 2011
- Mizoguchi H, Nakade J, Tachibana M, Ibi D, Someya E, Koike H, Kamei H, Nabeshima T, Itohara S, Takuma K, Sawada M, Sato J, Yamada K. Matrix metalloproteinase-9 contributes to kindlined seizure development in pentylenetetrazole-treated mice by converting pro-BDNF to mature BDNF in the hippocampus. J Neurosci., 2011; 31: 12963-12971.
- Kuroda K, Yamada S, Tanaka, Iizuka M, Yano H, Mori D, Tsuboi D, Nishioka T, Namba T, Iizuka Y, Kubota S, Nagai T, Ibi D, Wang R, Enomoto A, Isotani-Sakakibara M, Asai N, Kimura K, Kiyonari H, Abe T, Mizoguchi A, Sokabe M, Takahashi M, Yamada K, Kaibuchi K. Behavioral alterations associated with targeted disruption of exons 2 and 3 of the DISC1 gene in the mouse. Hum. Mol. Genet., 2011; 20: 4666-4683.
- Furukawa-Hibi Y, Alkam T, Nitta A, Matsuyama A, Mizoguchi H, Suzuki K, Moussaoui S, Yu Q-S, Greig NH, Nagai T, Yamada K: Butyrylcholinesterase inhibitors ameliorate cognitive dysfunction induced by amyloid-ß peptide in mice. Behav Brain Res., 2011; 225: 222-229.
- Nagai T, Kitahara Y, Ibi D, Nabeshima T, Sawa A, Yamada K: Effects of antipsychotics on the behavioral deficits in human dominant-negative DISC1 transgenic mice with neonatal polyI:C treatment. Behav. Brain Res., 2011; 225: 305-310.
- Ishizuka M, Fujimoto Y, Itoh Y, Sano M, Miyagawa Y, Ando A, Hiramatsu M, Hirasawa N, Ishihara S, Nabeshima T, Yamada K. Relationship between hematotoxicity and serum albumin level in the treatment of head and neck cancers with concurrent chemoradiotherapy using cisplatin. Jpn. J. Clin. Oncol., 2011; 41: 973-979.
- Furukawa-Hibi Y, Nitta A, Ikeda T, Morishita K, Liu W, Ibi D, Alkam T, Nabeshima T, Yamada K. The hydrophobic dipeptide Leu-Ile inhibits immobility induced by repeated forced swimming via the induction of BDNF. Behav Brain Res., 2011; 220: 271-280.
- Mizoguchi H, Ibi D, Takase F, Nagai T, Kamei H, Toth E, Sato J, Takuma K, Yamada K. Nicotine ameliorates impairment of working memory in methamphetamine-treated rats. Behav. Brain Res., 2011; 220: 159-163.
Research Keywords
Animal model, decision-making, drug addiction/dependence, emotion, learning and memory, mental disorders