Laboratories
- Back
- Top > Laboratories > Clinical Pharmacology > Biostatistics
Clinical PharmacologyBiostatistics
Introduction
Biostatistics aims to contribute to health sciences, through creating and practicing effective statistical methods to solve important statistical problems from a wide spectrum of biomedical researches.
The Department of Biostatistics at the Nagoya University Graduate School of Medicine is carrying out many methodological researches in biostatistics, including design and analysis of clinical trials and observational studies, disease clustering in spatial epidemiology, meta-analysis, analysis of genomic data, and so forth.
The faculty members are also engaged in many medical research projects and continuously bridging biostatistical design and analysis to a wide variety of statistical problems encountered in these projects. This also enables them to provide graduate students with the good practice of biostatistics. Our graduates are expected to have leadership careers as researchers and practitioners in academic biostatistics departments or data centers, government, and industry.
Research Projects
1. Statistical methodologies and their applications for clinical development of precision medicine:
Recent advances in biotechnology have accelerated the development of personalized or precision medicine, which uses patient-specific molecular characteristics to optimize diagnosis and treatment. To promote precision medicine, we must seek a new paradigm for the clinical development of therapeutics that incorporates diagnostics (e.g., molecular biomarkers) that are capable of identifying patient-specific treatment responses. The development of novel statistical methods for developing and validating diagnostics, testing of efficacy of treatments based on diagnostics, and evaluating clinical utility of treatments and diagnostics will help to achieve the paradigm shift in clinical studies. We expect that the methodologies that we create will significantly increase the likelihood of successfully bringing precision medicine to clinical practice.
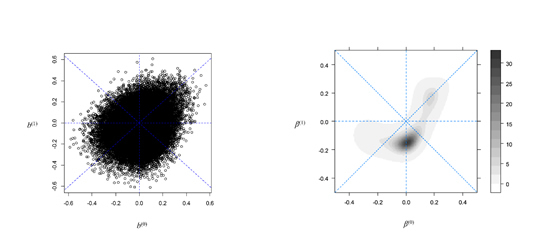
A) B)
Fig 1.1. Plots of estimated effects of microarray gene expressions on overall survival (in terms of log-hazard ratio) in thalidomide and control treatment groups for 50,000 or more genes (Panel A) and an estimate of the underlying effect size distribution using a semi-parametric hierarchical mixture model (Panel B) [1].
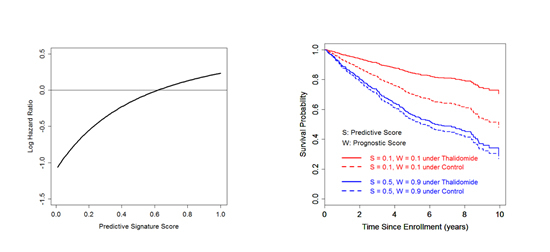
A) B)
Fig 1.2. Estimated profile of thalidomide’s effect as a function of a developed genomic predictive signature (Panel A) and estimated patient-level survival functions (Panel B) in multiple myeloma [13].
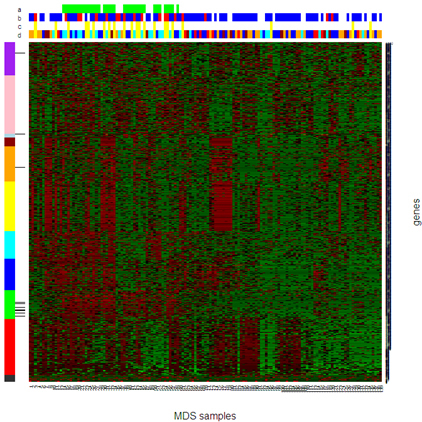
Fig 1.3. Nested two-way clustering (based on finite mixture models) of gene expression data from bone marrow mononuclear cells in myelodysplastic syndromes.
2. Statistical methods and their applications in spatial epidemiology:
With increasing public health concerns about environmental risk, the need for sophisticated methods for analyzing spatial health events is immediate to reveal spatial patterns not previously recognized. In particular, the research area of statistical methods for disease clustering now attracts a wide audience due to the perceived need to implement wide-ranging monitoring systems to detect possible health-related events such as the occurrence of infectious disease. For the cluster detection test, the spatial scan statistic is one of the most powerful approaches as it stands directly on a concrete statistical framework, which is flexible of different data types, such as purely spatial, temporal and spatio-temporal ones. The development of novel statistical methods for assessments of spatial variations of the risk of disease and health outcomes, will give powerful tools for studies in spatial epidemiology. We also develop a software and are exploring application thereof in various fields, such as surveying actual regional characteristics of diseases including intractable diseases, detection of disease related-genes, brain image analysis and signal detection based on spontaneous report of side effects brought on by pharmaceutical products.
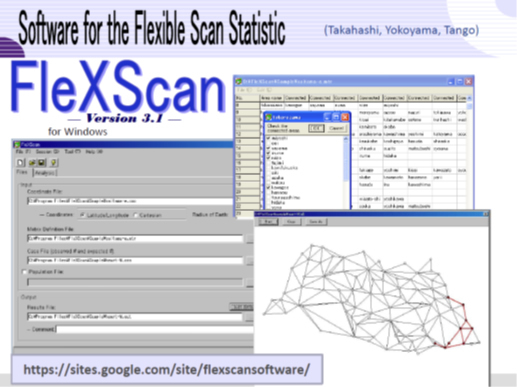
Fig 2.1. A software for disease clustering test, FleXScan (developed by Takahashi et al.)
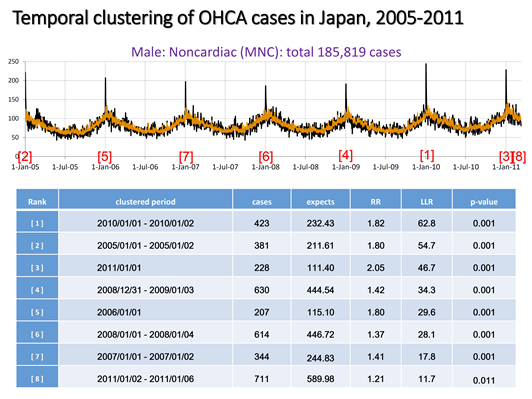
Fig 2.2. The daily out-of-hospital cardiac arrest (OHCA), male non-cardiac cases (MNC), in Japan from 1 January 2005 to 10 March 2011 (black solid line) with the null expected counts (orange line), along with significant temporal clusters detected by the spatial scan statistic (table). [6]
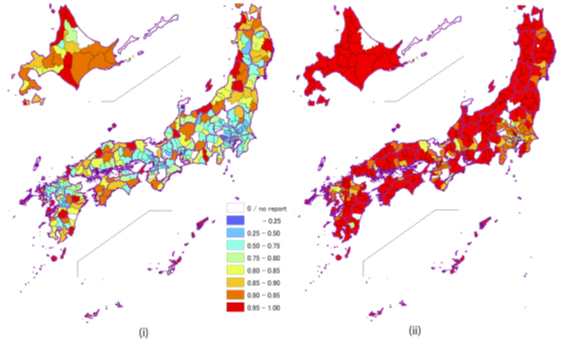
Fig 2.3. Maps of regional behavior of patients with mental disorders in Japan. Bayesian estimates of percentages of inpatients from (i) within the secondary medical area and (ii) within the prefecture. [2]
Faculty Members
| Faculty | Position | Department |
|---|---|---|
| Shigeyuki Matsui | Professor | Biostatistics |
| Kunihiko Takahashi | Associate Professor | Biostatistics |
| Takahiro Otani | Designated Assistant Professor | Biostatistics |
Bibliography
- 2019
- Matsui S, Igeta M, Toyoizumi K. Biomarker-based phase II and III clinical trials in oncology. In Textbook of Clinical Trials in Oncology. (eds. S. Halabi and S. Michiels), CRC Press. 2019. (In press).
- Takahashi K, Takahashi H, Nakaya T, Yasumura S, Ohira T, Ohto H, Ohtsuru A, Midorikawa S, Suzuki S, Shimura H, Yamashita S, Tanigawa K, Kamiya K. Factors influencing the proportion of non-examinees in the Fukushima Health Management Survey for childhood and adolescent thyroid cancer: Results from the baseline survey. Journal of Epidemiology, 2019. (in press)
- Otani T, Noma H, Sugasawa S, Kuchiba A, Goto A, Yamaji T, Kochi Y, Iwasaki M, Matsui S, Tsunoda T. Exploring predictive biomarkers from clinical genome-wide association studies via multidimensional hierarchical mixture models. European Journal of Human Genetics, 2019; 27: 140-149.
- Kawabata T, Emoto R, Nishino J, Takahashi K, Matsui S. Two-stage analysis for selecting fixed numbers of features in omics association studies. Statistics in Medicine, 2019; 38(16): 2956-2971.
- Emura T, Matsui S, Rondeau V. Survival Analysis with Correlated Endpoints: Joint Frailty-Copula Models, Springer, 2019. (In press).
- Nonaka T, Igeta, M, Matsui S. Statistical testing strategies for assessing treatment efficacy and marker accuracy in phase III trials. Pharmaceutical Statistics, 2019. (In press)
- Sakai Y, Honda M, Matsui S, Komori O, Murayama T, Fujiwara T, Mizuno M, Imai Y, Yoshimura K, Nasti A, Wada T, Iida N, Kitahara M, Horii R, Tamai T, Nishikawa M, Okafuji H, Mizukoshi E, Yamashita T, Yamashita T, Arai K, Kitamura K, Kawaguchi K, Takatori H, Shimakami T, Terashima T, Hayashi T, Nio K, Kaneko S; Hokuriku Liver Study Group. Development of novel diagnostic system for pancreas cancer including early stages measuring mRNA of whole blood cells. Cancer Science, 2019. (In press)
- Nishikimi M, Ogura T, Nishida K, Takahashi K, Nakamura M, Matsui S, Matsuda N, Iwami T. External validation of a risk classification at the emergency department of post-cardiac arrest syndrome patients undergoing targeted temperature management. Resuscitation, 2019. (in press)
- Okada E, Takahashi K, Nakamura K, Ukawa S, Takabayashi S, Nakamura M, Sasaki S, Tamakoshi A, Takimoto H. Dietary patterns and impaired abnormal glucose tolerance among Japanese: findings from the National Health and Nutrition Survey, 2012. Public Health Nutrition, 2019. (in press)
- Liu K, Ogura T, Takahashi K, Nakamura M, Ohtake H, Fujiduka K, Abe E, Oosaki H, Miyazaki D, Suzuki H, Nishikimi M, Lefor AK, Mato T. A progressive early mobilization program is significantly associated with clinical and economic improvement: A single-center quality comparison study. Critical Care Medicine, 2019. (in press)
- Kasugai D, Hirakawa A, Ozaki M, Nishida K, Ikeda T, Takahashi K, Matsui S, Uenishi N. Maximum norepinephrine dosage within 24 hours as an indicator of refractory septic shock: a retrospective study. Journal of Intensive Care Medicine 2019. (in press)
- Komori S, Ueno S, Ito Y, Sayo A, Meinert M, Kominami T, Inooka D, Kitagawa M, Nishida K, Takahashi K, Matsui S, Terasaki H. Steeper macular curvature in eyes with non-highly myopic retinitis pigmentosa. Investigative Ophthalmology & Visual Science 2019. (in press)
- Emura T, Matsui S, Chen HY. compound.Cox: univariate feature selection and compound covariate for predicting survival. Computer Methods and Programs in Biomedicine, 2019, 168: 21-37.
- Anzai T, Takahashi K, Watanabe M. Adverse reaction reports of neuroleptic malignant syndrome induced by atypical antipsychotic agents in the Japanese Adverse Drug Event Report (JADER) database. Psychiatry and Clinical Neurosciences, 2019; 73: 27-33.
- Nomura K, Hiyama TY, Sakuta H, Matsuda T, Lin CH, Kobayashi K, Kobayahi K, Kuwaki T, Takahashi K, Matsui S, Noda M. Na+ increases in body fluids sensed by central Na_x induce sympathetically mediated blood pressure elevations via H+-dependent activation of ASIC1a. Neuron, 2019; 101, 60-75.
- Kasugai D, Nishikimi M, Nishida K, Higashi M, Yamamoto T, Numaguchi A, Takahashi K, Matsui S, Matsuda N. Timing of administration of epinephrine predicts the responsiveness to epinephrine in norepinephrine-refractory septic shock: a retrospective study. Journal of Intensive Care, 2019; 7: 20.
- Yamauchi T, Sasaki T, Takahashi K, Umezaki S, Takahashi M, Yoshikawa T, Suka M, Yanagisawa H. Long working hours, sleep-related problems, and near-misses/injuries in industrial settings using a nationally representative sample of workers in Japan. PLoS ONE 2019; 14(7): e0219657.
- 2018
- Matsui S, Crowley J. Biomarker-stratified phase III clinical trials: enhancement with a subgroup-focused sequential design. Clinical Cancer Research, 2018; 24: 994-1001.
- Matsui S, Noma H, Qu P, Yoshio Sakai, Matsui K, Heuck C, Crowley J. Multi-subgroup gene screening using semi-parametric hierarchical mixture models and the optimal discovery procedure: application to a randomized clinical trial in multiple myeloma. Biometrics, 2018; 74: 313-320.
- Takahashi K, Shimadzu H. Multiple-cluster detection test for purely temporal disease clustering: Integration of scan statistics and generalized linear models. PLoS ONE, 2018; 13: e0207821.
- Otani T, Noma H, Nishino J, Matsui S. Re-assessment of multiple testing strategies for more efficient genome-wide association studies. European Journal of Human Genetics, 2018; 26: 1038-1048.
- Nishino J, Ochi H, Kochi Y, Tsunoda T, Matsui S. Sample size for successful genome-wide association study of major depressive disorder. Frontiers in Genetics 2018; 9: 227.
- Nishino J, Kochi Y, Shigemizu D, Kato M, Ikari K, Ochi H, Noma H, Matsui K, Morizono T, Boroevich K, Tsunoda T, Matsui S. Empirical Bayes estimation of semi-parametric hierarchical mixture models for unbiased characterization of polygenic disease architectures. Frontiers in Genetics, 2018; 9: 115.
- Igeta M, Takahashi K, Matsui S. Power and sample size calculation incorporating misspecifications of the variance function in comparative clinical trials with over-dispersed count data. Biometrics, 2018; 74: 1459-1467.
- Horisaki K, Takahashi K, Ito H, Matsui S. A dose-response meta-analysis of coffee consumption and colorectal cancer risk in the Japanese population: application of a cubic-spline model. 2018; Journal of Epidemiology,28: 503-509.
- Hirakawa A, Sato H, Daimon T, Matsui S. Modern Dose-Finding Designs for Cancer Phase I Trials: Drug Combination and Molecularly Targeted Agents. Springer, 2018.
- Kuriki S, Takahashi K, Hara H. Multiplicity adjustment for temporal and spatial scan statistics using Markov property. Japanese Journal of Statistics and Data Science, 2018; 1: 191-213.
- Nakaya T, Takahashi K, Takahashi H, Yasumura S, Ohira T, Ohto H, Ohtsuru A, Midorikawa S, Suzuki S, Shimura H, Yamashita S, Tanigawa K, Kamiya K. Spatial analysis of the geographical distribution of thyroid cancer cases from the first-round thyroid ultrasound examination in Fukushima Prefecture. Scientific Reports, 2018; 8: 17661.
- Liu K, Ogura T, Takahashi K, Nakamura M, Ohtake H, Fujiduka K, Abe E, Oosaki H, Miyazaki D, Suzuki H, Nishikimi M, Kawarai-Kefor A, Mato T. The safety of a novel early mobilization protocol conducted by ICU physicians: a prospective observational study. Journal of Intensive Care, 2018; 6: 10.
- Nishikimi M, Ogura T, Matsui K, Takahashi K, Fukaya K, Liu K, Morita H, Nakamura M, Matsui S, Matsuda N. Accuracy of the first interpretation of early brain CT images for predicting the prognosis of post-cardiac arrest syndrome patients at the emergency department. Journal of Intensive Care, 2018; 6: 26.
- Okumura J, Shindo Y, Takahashi K, Sano M, Sugino Y, Yagi T, Taniguchi H, Saka H, Matsui S, Hasegawa Y. Mortality in patients with community-onset pneumonia at low risk of drug-resistant pathogens: Impact of β-lactam plus macrolide combination therapy. Respirology, 2018; 23: 526-534.
- Nishikimi M, Ogura T, Nishida K, Takahashi K, Fukaya K, Liu K, Nakamura M, Matsui S, Matsuda N. Differential effect of mild therapeutic hypothermia depending on the findings of hypoxic encephalopathy on early CT images in patients with post-cardiac arrest syndrome. Resuscitation, 2018; 128: 11-15.
- Nishikimi M, Numaguchi A, Takahashi K, Miyagawa Y, Matsui K, Higashi M, Makishi G, Matsui S, Matsuda N. Effect of administration of Ramelteon, a melatonin receptor agonist, on the duration of stay in the ICU: a single center randomized placebo-controlled trial. Critical Care Medicine, 2018; 46: 1099-1105.
- Fukamachi S, Hashiguchi M, Takahashi K, Mochizuki M. Exploratory evaluation of a commercially available Japanese medical record database as an external control for comparative clinical trials in new drug development: Amyotrophic lateral sclerosis. Neurology and Clinical Neuroscience, 2018; 6: 94-99.
- Tominaga Y, Aomori T, Hayakawa T, Kijima N, Morisky DE, Takahashi K, Mochizuki M. Possible associations of personality traits representing harm avoidance and self-directedness with medication adherence in Japanese patients with type 2 diabetes. Journal of Pharmaceutical Health Care and Sciences, 2018; 4: 16.
- Matsuhisa T, Takahashi N, Aomatsu M, Takahashi K, Nishino J, Ban N, Mercer SW. How many patients are required to provide a high level of reliability in the Japanese version of the CARE Measure? A secondary analysis. BMC Family Practice, 2018; 19: 138.
- Ogura T, Nakamura Y, Takahashi K, Nishida K, Kobashi D, Matsui S. Treatment of patients with sepsis in a closed Intensive Care Unit is associated with improved survival: A nationwide observational study in Japan. Journal of Intensive Care, 2018; 6: 57.
- Tominaga Y, Aomori T, Hayakawa T, Morisky DE, Takahashi K, Mochizuki M. Relationship between medication adherence and glycemic control in Japanese patients with type 2 diabetes. Pharmazie, 2018; 73: 609-612.
- Okada E, Takahashi K, Takimoto H, Takabayashi S, Kishi T, Kobayashi T, Nakamura K, Ukawa S, Nakamura M, Sasaki S, Tamakoshi A. Dietary patterns among Japanese adults: findings from the National Health and Nutrition Survey, 2012. Asia Pacific Journal of Clinical Nutrition, 2018; 27: 1120-1130.
- Shimamura F, Hamada C, Matsui S, Hirakawa A. Two-stage approach based on zone and dose findings for two-agent combination phase I/II trials. Journal of Biopharmaceutical Statistics, 2018; 8: 1-13.
- Emura T, Nakatochi M, Matsui S, Michimae H, Rondeau V. Personalized dynamic prediction of death according to tumour progression and high-dimensional genetic factors: Meta-analysis with a joint model. Statistical Methods in Medical Research, 2018; 27: 2842-2858.
- Funahashi S, Okazaki Y, Nishiyama T, Ohyoshi H, Yasui H, Nishida K, Matsui S, Toyokuni S. Global overexpression of divalent metal transporter 1 delays crocidolite-induced mesothelial carcinogenesis in male mice. Free Radical Research, 2018; 52: 1030-1039.
- Komura T, Yano M, Miyake A, Takabatake H, Miyazawa M, Ogawa N, Seki A, Honda M, Wada T, Matsui S, Kaneko S, Sakai Y. Immune condition of colorectal cancer patients featured by serum chemokines and gene expressions of CD4+ cells in blood. Canadian Journal of Gastroenterology and Hepatology, 2018; 7436205.
- Hayashi K, Oshima H, Shimizu M, Kobayashi K, Matsui S, Nishida Y, Usui A. Preoperative six-minute walk distance is associated with postoperative cognitive dysfunction. Annals of Thoracic Surgery, 2018; 106: 505-512.
- Kawai A, Goto T, Shibata T, Tani K, Mizutani S, Nishikawa A, Shibata T, Matsumoto S, Nagata K, Narukawa M, Matsui S, Ando M, Toguchida J, Monden M, Heike T, Kimura S, Ueda R. The current state of therapeutic development for rare cancers in Japan, and proposals for improvement. Cancer Science, 2018; 109: 1731-1737.
- 2017
- Matsui S, Crowley J (editors). Frontiers of Biostatistical Methods and Applications in Clinical Oncology, Springer, 2017.
- Matsui S, Phase III clinical trial designs incorporating predictive biomarkers: an overview. In Frontiers of Biostatistical Methods and Applications in Clinical Oncology. (eds. S. Matsui, J. Crowley), Springer, 2017.
- Takahashi K, Tachimori H, Kan C, Nishi D, Okumura Y, Kato N, Takeshima T. Spatial analysis for regional behavior of patients with mental disorders in Japan. Psychiatry and Clinical Neurosciences, 2017; 71; 254-261.
- Toyoizumi K, Matsui S. Correcting estimation bias in randomized clinical trials with a test of treatment-by-biomarker interaction. Statistics in Biopharmaceutical Research, 2017; 9: 172-179.
- Noma H, Tanaka S, Matsui S, Cipriani A, Furukawa TA. Quantifying indirect evidence in network meta-analysis. Statistics in Medicine, 2017; 36: 917-927.
- Sugasawa S, Noma H, Otani T, Nishino J, Matsui S. An efficient and flexible test for rare variant effects. European Journal of Human Genetics, 2017; 25: 752-757.
- Nishikimi M, Matsuda N, Matsui K, Takahashi K, Ejima T, Liu K, Ogura T, Higashi M, Umino H, Makishi G, Numaguchi A, Matsushima S, Tokuyama H, Nakamura M, Matsui S. A novel scoreing system for predicting the neurological prognosis prior to the initiation of induced hypothermia in cases of post-cardiac arrest syndrome: the CAST score. Journal of Trauma, Resuscitation and Emergency Medicine, 2017; 25: 49.
- Takagishi M, Sawada M, Ohata S, Asai N, Enomoto A, Takahashi K, Weng L, Ushida K, Ara H, Matsui S, Kaibuchi K, Sawamoto K, Takahashi M. Daple coordinates 1 planar polarized microtubule dynamics in ependymal cells and contributes to hydrocephalus. Cell Reports, 2017; 20: 960-972.
- Sayo A, Ueno S, Kominami T, Nishida K, Inooka D, Nakanishi A, Yasuda S, Okado S, Takahashi K, Matsui S, Terasaki H. Longitudinal study of visual field changes determined by Humphrey Field Analyzer 10-2 in patients with Retinitis Pigmentosa. Scientific Reports, 2017; 7: 16383.
- Takahashi H, Takahashi K, Shimura H, Yasumura S, Suzuki S, Ohtsuru A, Midorikawa S, Ohira T, Ohto H, Yamashita S, Kamiya K. Simulation of expected childhood and adolescent thyroid cancer cases in Japan using a cancer-progression model based on the National Cancer Registry: application to the first-round thyroid examination of the Fukushima Health Management Survey. Medicine, 2017; 96: e8631.
- Okumura Y, Sakata N, Takahashi K, Nishi D, Tachimori H. Epidemiology of overdose episodes from the period prior to hospitalization for drug poisoning until discharge in Japan: an exploratory descriptive study using a nationwide claims database. Journal of Epidemiology, 2017; 27: 373-380.
- 2016
- Nishikimi M, Matsuda N, Matsui K, Takahashi K, Ejima T, Liu K, Ogura T, Higashi M, Umino H, Makishi G, Numaguchi A, Matsushima S, Tokuyama H, Nakamura M, Matsui S. CAST: a new score for early prediction of neurological outcomes after cardiac arrest before therapeutic hypothermia with high accuracy. Intensive Care Medicine, 2016; 42: 2106-2107.
- Sadashima E, Hattori S, Takahashi K. Meta-analysis of prognostic studies for a biomarker with a study-specific cut-off value. Research Synthesis Methods, 2017; 7: 402-419.
- Ishikawa T, Uetake H, Murotani K, Kobunai T, Ishiguro M, Matsui S, Sugihara K. Genome-wide DNA copy-number analysis in ACTS-CC trial of adjuvant chemotherapy for stage III colonic cancer. Anticancer Research, 2016; 36: 853-860.
- Kobayashi M, Iwase T, Yamamoto K, Ra E, Murotani K, Matsui S, Terasaki H. Association between photoreceptor regeneration and visual acuity following surgery for rhegmatogenous retinal detachment. Investigative Ophthalmology & Visual Science, 2016; 57: 889-898.
- 2015
- Matsui S, Buyse M, Simon R. (editors). Design and Analysis of Clinical Trials for Predictive Medicine. CRC Press, 2015.
- Matsui S, Choai Y, Nonaka T. Phase III all-comers clinical trials with a predictive biomarker. In Design and Analysis of Clinical Trials for Predictive Medicine. (eds. S. Matsui, M. Buyse, R. Simon), CRC Press, 2015.
- Matsui S. Statistical issues in clinical development and validation of genomic signatures. In Design and Analysis of Clinical Trials for Predictive Medicine. (eds. S. Matsui, M. Buyse, R. Simon), CRC Press, 2015.
- Matsui S. Development and validation of continuous genomic signatures in randomized clinical trials. In Design and Analysis of Clinical Trials for Predictive Medicine. (eds. S. Matsui, M. Buyse, R. Simon), CRC Press, 2015.
- Simon R, Matsui S, Buyse M. Clinical trials for predictive medicine: new paradigms and challenges. In Design and Analysis of Clinical Trials for Predictive Medicine. (eds. S. Matsui, M. Buyse, R. Simon), CRC Press, 2015.
- Daimon T, Hirakawa A, Matsui S. Phase I dose-finding designs and their applicability to targeted therapies. In Design and Analysis of Clinical Trials for Predictive Medicine. (eds. S. Matsui, M. Buyse, R. Simon), CRC Press, 2015.
- Noma H, Matsui S. Univariate analysis for gene screening: beyond the multiple testing. In Design and Analysis of Clinical Trials for Predictive Medicine. (eds. S. Matsui, M. Buyse, R. Simon), CRC Press, 2015.
- Takahashi K, Shimadzu H. The daily incidence of out-of-hospital cardiac arrest unexpectedly increases around New Yesar’s Day in Japan. Resuscitation, 2015; 96: 156-162.
- Choai Y, Matsui S. Estimation of treatment effects in all-comers randomized clinical trials with a predictive marker. Biometrics, 2015; 71: 25-32.
- Nomura K, Takahashi K, Hinomura Y, Kawaguchi G, Matsushita Y, Marui H, Anzai T, Hashiguchi M, Mochizuki M. Effect of database profile variation on drug safety assessment: an analysis of spontaneous adverse event reports of Japanese cases. Drug Design, Development and Therapy, 2015; 9: 3031-3041.
- Hirakawa A, Wages NA, Sato H, Matsui S. A comparative study of adaptive dose-finding designs for phase I oncology trials of combination therapies. Statistics in Medicine, 2015; 34: 3194-3213.
- Hirakawa A, Matsui S. Operating characteristics of restrictions on skipping dose level for adaptive dose-finding method in two-agent phase I trials. Japanese Journal of Biometrics, 2015; 36: 1-12.
- Yanagida K, Iwase T, Yamamoto K, Ra E, Kaneko H, Murotani K, Matsui S, Terasaki H. Sex-related differences in ocular blood flow of healthy subjects using laser speckle flowgraphy. Investigative Ophthalmology & Visual Science, 2015; 56: 4880-4890.
- Sugimoto T, Matsumoto T, Hosoi T, Miki T, Gorai I, Yoshikawa H, Tanaka Y, Tanaka S, Fukunaga M, Sone T, Nakano T, Ito M, Matsui S, Yoneda T, Takami H, Watanabe K, Osakabe T, Okubo N, Shiraki M, Nakamura T. Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT). Osteoporosis International, 2015; 26: 765-774.
- Takanari K, Araki Y, Okamoto S, Sato H, Yagi S, Toriyama K, Yokotyama K, Murotani K, Matsui S, Wakabayashi T, Kamei Y. Operative wound related complications after cranial revascularization surgeries. Journal of Neurosurgery, 2015; 123: 1145-1150.
- Iwase T, Yamamoto K, Ra E, Murotani K, Matsui S, Terasaki H. Diurnal variations in blood flow at optic nerve head and choroid in healthy eyes: diurnal variations in blood flow. Medicine, 2015; 94: e519.
- 2014
- Matsui S, Choai Y, Nonaka T. Comparison of statistical analysis plans in randomize-all phase III trials with a predictive biomarker. Clinical Cancer Research, 2014; 20: 2820-2830.
- Kubokawa T, Hasukawa M, Takahashi K. On measuring uncertainty of benchmarked predictors with application to disease risk estimate. Scandinavian Journal of Statistics, 2014; 41: 394-413.
- Ogino D, Takahashi K, Sato H. Characteristics of clinical trial websites: information distribution between ClinicalTrials.gov and 13 primary registries in the WHO registry network. Trials, 2014; 15: 428.
- 2013
- Matsui S. Genomic biomarkers for personalized medicine: development and validation in clinical studies. Computational and Mathematical Methods in Medicine, 2013; Article ID: 865980.
- Takahashi K, Nakao H, Hattori S. Cubic spline regression of J-shaped dose-response curves with likelihood-based assignments of grouped exposure levels. Journal of Biometrics & Biostatistics, 2013; 4: 181.
- 2012
- Matsui S, Simon R, Qu P, Shaughnessy J, Barlogie B, Crowley J. Developing and validating continuous genomic signatures in randomized clinical trials for predictive medicine. Clinical Cancer Research, 2012; 18: 6065-6073.
- Tango T, Takahashi K. A flexible spatial scan statistic with a restricted likelihood ratio for detecting disease clusters. Statistics in Medicine, 2012; 31: 4207-4218.
- 2011
- Matsui S, Noma H. Estimation and selection in high-dimensional genomic studies for developing molecular diagnostics. Biostatistics, 2011; 12: 223-233.
- Matsui S, Noma H. Estimating effect sizes of differentially expressed genes for power and sample size assessments in microarray experiments. Biometrics, 2011; 67: 1225-1235.
- Nishiyama T, Takahashi K, Tango T, Pinto D, Scherer SW, Takami S, Kishino H. A scan statistic to extract causal gene clusters from case-control genome-wide rare CNV data. BMC Bioinformatics, 2011; 12: 205.
- Tango T, Takahashi K, Kohriyama K. A space-time scan statistic for detecting emerging outbreaks. Biometrics, 2011; 67: 106-115.
other
Candidates of graduate students in biostatistics should have a basis of mathematical statistics at least in an undergraduate level, as well as being interested in applications of statistical methods in biomedical studies.