Laboratories
- Back
- Top > Laboratories > High-Technology Application of Medicine > Clinical Oncology and Chemotherapy
High-Technology Application of MedicineClinical Oncology and Chemotherapy
Introduction
The Department of Clinical Oncology and Chemotherapy, established in 2005, is a clinical department that performs high-level outpatient pharmacotherapy for malignant neoplasms in all organs, based on close collaboration with each of the other clinical departments. The department is regularly consulted by other clinical departments on matters related to chemotherapy and palliative care.
Treatment with antineoplastic agents requires more than just familiarity with anticancer drugs and knowledge of protocols. In addition to an understanding of the clinical pharmacology of each drug and its series of adverse effects, high-level cancer pharmacology demands intimate knowledge of the pathophysiology of malignant neoplasms that occur specifically in each organ. It also demands advanced clinical skill in judging the advantages and disadvantages of various regimens depending on the response to treatment and changes in condition of individual patients.
As a result of the enthusiasm for early-phase clinical trials of anticancer agents under development, the department has been entrusted with industry-sponsored clinical trials for registration, including investigating agents that had never been administered in Japanese subjects.
Research Projects
1. Appropriate anticancer agent therapy based on clinical pharmacology
We study the appropriate cancer pharmacotherapy for the patients requiring special consideration, including patients with organ dysfunction, elderly patients and patients with sarcopenia.
i). Correlation of free carboplatin clearance with pretreatment GFR in Japanese patients, validation study of Calvert formula (ASCO 2009, bibliography 25).
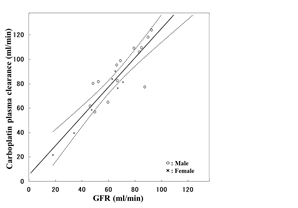
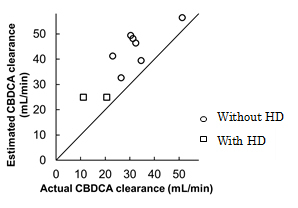
ii). Correlation of free carboplatin clearance with pretreatment GFR in patients with renal dysfunction (ESMO 2014, bibliography 12).
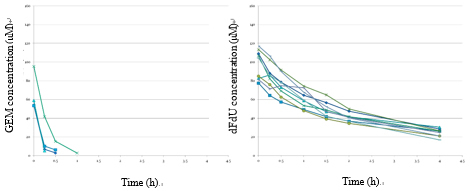
iii). Pharmacokinetic analysis of gemcitabine in patients with hepatic dysfunction (JSMO 2013, bibliography 7).
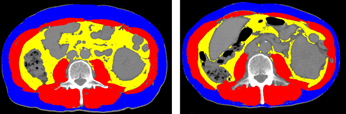
iv). Body composition change after long-term use of molecular targeted agents (JSMO 2016, bibliography 5).
Baseline 4 months later
2. Individualized anticancer agent therapy based on pharmacogenetics
We study the association between various genetic polymorphism and effect or side effect of the cancer pharmacotherapy.
3. Early detection of anticancer drug’s side effect
We conduct exploratory clinical studies to discover side effects of anticancer drugs at an early stage.
4. Clinical trials in palliative medicine
We work on creation of the evidence in palliative medicine including a placebo-controlled randomized trial.
5. Early development trial of new anticancer drug
We perform the early development trial of new anticancer drugs such as molecular target agents, immune checkpoint inhibitors and so on.
Faculty Members
| Faculty | Position | Department |
|---|---|---|
| Yuchi Ando | Professor | Clinical Oncology and Chemotherapy |
| Ayako Mitsuma | Designated Associate Professor | Clinical Oncology and Chemotherapy |
| Osamu Maeda | Designated Associate Professor | Clinical Oncology and Chemotherapy |
| Mihoko Sugishita | Assistant Professor | Clinical Oncology and Chemotherapy |
| Yasunori Adachi | Assistant Professor | Clinical Oncology and Chemotherapy |
| Tomoya Shimokata | Designated Assistant Professor | Clinical Oncology and Chemotherapy |
| Takefumi Mizutani | Designated Assistant Professor | Clinical Oncology and Chemotherapy |
Bibliography
- 2018
- Bishal Gyawali, Tomoya Shimokata, Kazunori Honda, Yuichi Ando. Reporting harms more transparently in trials of cancer drugs. BMJ 2018; 363 doi: https://doi.org/10.1136/bmj.k4383 (Published 01 November 2018)
- Naomi Hayashi, Ayumu Matsuoka, Hidemi Goto, Momokazu Gotoh, Hitoshi Kiyoi, Yasuhiro Kodera, Masato Nagino, Fumio Nagashima, Yuichi Ando. Clinical effectiveness of geriatric assessment for predicting the tolerability of outpatient chemotherapy in older adults with cancer. J Geriatr Oncol. 2018 Jan;9(1):84-86. doi: 10.1016/j.jgo.2017.07.014. Epub 2017 Aug 9.
- Sachi Morita, Toyone Kikumori, Nobuyuki Tsunoda, Takahiro Inaishi, Yayoi Adachi, Akiko Ota, Masahiro Shibata, Ayumu Matsuoka, Kenichi Nakanishi, Dai Takeuchi, Takehumi Mizutani, Tomoya Shimokata, Hiromichi Hayashi, Osamu Maeda and Yuichi Ando. Feasibility of dose-dense epirubicin and cyclophosphamide with subcutaneous pegfilgrastim 3.6 mg support: a single-center prospective study in Japan. Int J Clin Oncol. 2018 Feb;23(1):195-200. doi: 10.1007/s10147-017-1177-z. Epub 2017 Aug 8.
- Horie Shigeo, Mototsugu Oya, Masaomi Nangaku, Yoshinari Yasuda, Yasuhiro Komatsu, Motoko Yanagita, Yuko Kitagawa, Hiroyuki Kuwano, Hiroyuki Nishiyama, Chikashi Ishioka, Hiromasa Takaishi, Hideki Shimodaira, Akira Mogi, Yuichi Ando, Koji Matsumoto, Daisuke Kadowaki, Satoru Muto. Guidelines for treatment of renal injury during cancer chemotherapy 2016. Clin Exp Nephrol. 2018 Feb;22(1):210-244. doi: 10.1007/s10157-017-1448-z.
- 2017
- Hiroaki Tsukuura, Masayuki Miyazaki, Tatsuya Morita, Mihoko Sugishita, Hiroshi Kato, Yuka Murasaki, Bishal Gyawali, Yoko Kubo, Masahiko Ando, Masashi Kondo, Kiyofumi Yamada, Yoshinori Hasegawa and Yuichi Ando. Efficacy of Prophylactic Treatment for Oxycodone-Induced Nausea and Vomiting Among Patients with Cancer Pain (POINT): A Randomized, Placebo-Controlled, Double-Blind Trial.2018 Mar;23(3):367-374. doi: 10.1634/theoncologist.2017-0225. Epub 2017 Oct 16.
- Osamu Maeda, Ayumu Matsuoka, Ryoji Miyahara, Kohei Funasaka, Yoshiki Hirooka, Masahide Fukaya, Masato Nagino, Yasuhiro Kodera, Hidemi Goto and Yuichi Ando. Modified docetaxel, cisplatin and capecitabine for stage IV gastric cancer in Japanese patients: A feasibility study. World J Gastroenterol. 2017 Feb 14;23(6):1090-1097. doi: 10.3748/wjg.v23.i6.1090.
- Bishal Gyawali, Tomoya Shimokata, Masahiko Ando, Kazunori Honda and Yuichi Ando. Risk of serious adverse events and fatal adverse events with sorafenib in patients with solid cancer: a meta-analysis of phase 3 randomized controlled trials. Ann Oncol. 2017 Feb 1;28(2):246-253. doi: 10.1093/annonc/mdw549.
- 2016
- Ayumu Matsuoka, Ayako Mitsuma, Osamu Maeda, Hiroaki Kajiyama, Hitoshi Kiyoi, Yasuhiro Kodera, Masato Nagino, Hidemi Goto, Yuichi Ando. Quantitative assessment of chemotherapy-induced peripheral neurotoxicity using a point-of-care nerve conduction device. Cancer Sci, 2016 doi: 10.1111/cas.13010.
- Bishal Gyawali, Tomoya Shimokata, Kazunori Honda, Hiroaki Tsukuura, Yuichi Ando. Should low-income countries invest in breast cancer screening? Cancer Causes Control, doi 10.1007/s10552-016-0812-8
- Hironobu Minami, Yuichi Ando, Brigette Buig Yue Ma, Jih-Hsiang Lee, Hiroyuki Momota, Yutaka Fujiwara, Leung Li, Koichi Fukino, Koji Ito, Takeshi Tajima, Asuka Mori, Chia-Chi Lin. Phase I, multicenter, open-label, dose-escalation study of sonidegib in Asian patients with advanced solid tumors. Cancer Sci, 2016; doi: 10.1111/cas.13022
- Kazunori Honda, Kyosuke Takeshita, Kenta Murotani, Ayako Mitsuma, Hironori Hayashi, Nobuyuki Tsunoda, Toyone Kikumori, Toyoaki Murohora, Yuichi Ando. Assessment of left ventricular diastolic function during trastuzumab treatment in patietns with HER2-positive breast cancer. Breast Cancer, 2016; doi: 10.1007/s12282-016-0705-4
- Bishal Gyawali, Tomoya Shimokata, Kazunori Honda, Chihiro Kondoh, Naomi Hayashi, Yasushi Yoshino, Naoto Sassa, Yasuyuki Nakano, Momokazu Gotoh, Yuichi Ando. Muscle wasting associated with the long term use of mTOR inhibitors. Mol Clin Oncol, 2016; 5: 641-646
- Koichiro Watanabe, Satoshi Otsu, Yoshinori Hirashima, Ryotaro Morinaga, Kazuo Nishikawa, Yasushi Hisamatsu, Tomoya Shimokata, Megumi Inada-Inoue, Takashi Shibata, Hiromi Takeuchi, Takahiro Watanabe, Kota Tokushige, Heiko Maacke, Kuniaki Shirao, Yuichi Ando. A phase I study of binimetinib (MEK162) in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol, 2016; 77: 1157-1164
- Takashi Shibata, Tomoki Ebata, Ken-ichi Fujita, Tomoya Shimokata, Osamu Maeda, Ayako Mitsuma, Yasutsuna Sasaki, Masato Nagino, Yuichi Ando. Optimal dose of gemcitabine for the treatment of biliary tract or pancreatic cancer in patients with liver dysfunction. Cancer Sci, 2016; 107: 168-172
- Naomi Hayashi, Yuichi Ando, Bishal Gyawali, Tomoya Shimokata, Osamu Maeda, Masahide Fukaya, Hidemi Goto, Masato Nagino, Yasuhiro Kodera. Low skeletal muscle density is associated with poor survival in patients who receive chemotherapy for metastatic gastric cancer. Oncol Rep, 2016; 35: 1727-1731
- Mihoko Sugishita, Tsuneo Imai, Toyone Kikumori, Ayako Mitsuma, Tomoya Shimokata, Takashi Shibata, Sachi Morita, Megumi Inada-Inoue, Masataka Sawaki, Yoshinori Hasegawa, Yuichi Ando. Pharmacogenetic association between GSTP1 genetic polymorphism and febrile neutropenia in Japanese patients with early breast cancer. Breast Cancer, 2016; 23: 195-201
- 2015
- Hiroaki Tsukuura, Yuichi Ando, Bishal Gyawali, Masami Matsumoto, Mihoko Sugishita, Kazunori Honda, Hiroshi Urakawa, Osamu Maeda, Yoshinori Hasegawa. Prophilactic use of antiemetics for prevention of opioid-induced nausea and vomiting: a questionnaire survey among Japanese Physicians. J Palliat Med, 2015; 18: 977-980
- Hirofumi Mukai, Norikazu Masuda, Hiroshi Ishiguro, Ayako Mitsuma, Takashi Shibata, Jun Yamamura, Masakazu Toi, Aiko Watabe, Akiko Sarashina, Martina Uttenreuther-Fischer, Yuihi Ando. Phase I trial of afatinib plus vinorelbine in Japanese patients with advanced solid tumors, including breast cancer. Cancer Chemother Pharmacol, 2015; 76: 739-750
- Tomoyo Oguri, Tomoya Shimokata, Isao Ito, Yoshinari Yasuda, Naoto Sassa, Masami Nishiyama, Akinobu Hamada, Yoshinori Hasegawa, Yuichi Ando. Extension of the Calvert formula to patients with severe renal insufficiency. Cancer Chemother Pharmacol, 2015; 76: 53-59
- Kanako Shibata, Yoshinari Yasuda, Ryo Kobayashi, Yuichi Ando, Tomoya Shimokata, Hideki Kamiya, Mutsuharu Hayashi, Shoichi Maruyama, Seiichi Matsuo, Makoto Nakao, Teruo Tsuchiya, Hitomi Teramachi. Renal function evaluation in patients with cancer who were scheduled to receive carboplatin or S-1. Clin Exp Nephrol, 2015; 19: 1107-1113
- 2014
- Megumi Inada-Inoue, Yuichi Ando, Kenji Kawada, Ayako Mitsuma, Masataka Sawaki, Taro Yokoyama, Yu Sunakawa, Hiroo Ishida, Kazuhiro Araki, Keishi Yamashita, Keiko Mizuno, Fumio Nagashima, Akiko Takekura, Kazuo Nagamatsu, Yasutsuna Sasaki. Phase 1 study of pazopanib alone or combined with lapatinib in Japanese patients with solid tumors. Cancer Chemother Pharmacol, 2014; 73: 673-683
- Yuichi Ando, Megumi Inada-inoue, Ayako Mitsuma, Takayuki Yoshino, Atsushi Ohtsu, Naoko Suenaga, Masahiko Sato, Tomoyuki Kakizume, Matthew Robson, Cornelia Quadt and Toshihiko Doi. A Phase I dose-escalation study of buparlisib (BKM120), an oral pan-class I PI3K inhibitor, in Japanese patients with advanced solid tumors. Cancer Sci, 2014; 105: 347-353
- 2013
- Tomoyo Oguri, Ayako Mitsuma, Megumi Inada-Inoue, Sachi Morita, Takashi Shibata, Tomoya Shimokata, Mihoko Sugishita, Goro Nakayama, Keisuke Uehara, Yoshinori Hasegawa and Yuichi Ando. Genetic polymorphisms associated with oxaliplatin-induced peripheral neurotoxicity in Japanese patients with colorectal cancer. Int J Clin Pharmacol Ther, 2013; 51: 475-481
- Yutaka Fujiwara, Yuichi Ando, Toru Mukohara, Naomi Kiyota, Naoko Chayahara, Ayako Mitsuma, Megumi Inada-Inoue, Masataka Sawaki, Robert Ilaria Jr., P. Kellie Turner, Jumpei Funai, Kaijiro Maeda and Hironobu Minami. A phase I study of tasisulam sodium using an albumin-tailored dose in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol, 2013; 71: 991-998
- Takashi Shibata, Yosuke Minami, Ayako Mitsuma, Sachi Morita, Megumi Inada-Inoue, Tomoyo Oguri, Tomoya Shimokata, Mihoko Sugishita, Tomoki Naoe and Yuichi Ando. Association between severe toxicity of nilotinib and UGT1A1 polymorphisms in Japanese patients with chronic myelogenous leukemia. Int J Clin Oncol, 2014; 19: 391-396
- Sachi Morita, Keisuke Uehara, Goro Nakayama, Megumi Inoue, Ayako Mitsuma, Tomoya Shimokata, Mihoko Sugishita, Takashi Shibata, Tomoyo Oguri, Yuichi Ando. Association between bevacizumab-related hypertension and VEGF gene polymorphisms in Japanese patients with metastatic colorectal cancer. Cancer Chemother Pharmacol, 2013; 71: 405-411
- 2012
- Yuichi Ando, Kenji Kawada, Megumi Inada, Sachi Morita, Ayako Mitsuma, Yoshinari Yasuda, Mariko Hiramatsu, Yasushi Fujimoto, Ken-ichi Fujita. Pharmacokinetic study of S-1 in patients in whom inulin clearance was measured. Oncology, 2012; 83: 38–44
- Sachi Morita, Koichi Kitagawa, Megumi Inada, Ayako Mitsuma, Masataka Sawaki, Satoshi Oizumi, Yoshito Komatsu, Satoshi Yuki, Hironobu Minami, Yutaka Fujiwara, Naomi Kiyota, Hiromi Tanii, Junko Kimura, Yuichi Ando. Phase I dose-escalating study of panobinostat (LBH589) administered intravenously to Japanese patients with advanced solid tumors. Invest New Drugs, 2012; 30: 1950–1957
- Koichi Kitagawa, Kenji Kawada, Sachi Morita, Megumi Inada, Ayako Mitsuma, Masataka Sawaki, Shigeo Iino, Yasuya Inden, Toyoaki Murohara, Tsuneo Imai and Yuichi Ando. Prospective evaluation of corrected QT intervals and arrhythmias after exposure to epirubicin, cyclophosphamide, and 5-fluorouracil in women with breast cancer. Ann Oncol, 2012; 23: 74 3-747
- Kazuhiro Araki, Koichi Kitagawa, Hirofumi Mukai, Toru Mukohara, Keiji Kodama, Yuichi Ando, Masaru Narabayashi, Hironobu Minami, Kiyomi Mera and Yasutsuna Sasaki. First clinical pharmacokinetic dose-escalation study of sagopilone, a novel, fully synthetic epothilone, in Japanese patients with refractory solid tumors. Invest New Drugs, 2012; 30: 2327-2333
- 2010
- Megumi Inada, Mitsuo Sato, Sachi Morita, Koichi Kitagawa, Kenji Kawada, Ayako Mitsuma, Masataka Sawaki, Ken-ichi Fujita and Yuichi Ando. Associations between oxaliplatin-induced peripheral neuropathy and polymorphisms of the ERCC1 and GSTP1 genes. Int J Clin Pharmacol Ther, 2010; 48: 729-734
- Tomoya Shimokata, Yuichi Ando, Yoshinari Yasuda, Akinobu Hamada, Kenji Kawada, Hideyuki Saito, Seiichi Matsuo, Masashi Kondo, Kazuyoshi Imaizumi, Yoshinori Hasegawa. Prospective evaluation of pharmacokinetically guided dosing of carboplatin in Japanese patients with cancer. Cancer Sci, 2010; 101: 2601-2605
- Tomoyo Oguri, Tomoya Shimokata, Megumi Inada, Isao Ito, Yuichi Ando, Yasutsuna Sasaki, Yoshinori Hasegawa. Pharmacokinetic analysis of carboplatin in patients with cancer who are undergoing hemodialysis. Cancer Chemother Pharmacol, 2010; 66: 813-817
- Kenji Kawada, Koichi Kitagawa, Sachi Kamei, Megumi Inada, Ayako Mitsuma, Masataka Sawaki, Toyone Kikumori, Yasushi Fujimoto, Hiroshi Arima, Tsuneo Imai and Yuichi Ando. The feasibility study of docetaxel in patients with anaplastic thyroid cancer. Jpn J Clin Oncol, 2010; 40: 596-599
Research Keywords
Clinical Oncology, Clinical Pharmacology, Pharmacogenomics, Patient Safety, Outpatient Chemotherapy, Palliative Care
Call for graduate students
The curriculum outlines the broad concepts, related learning objectives and the associated theoretical knowledge, clinical skills, attitudes and behaviors required and commonly utilized by medical oncology physicians within and outside Japan. At the completion of the PhD in the program, students should be competent to provide at consultant level, unsupervised comprehensive medical care in medical oncology.
The training includes aspects of basic science and internal medicine course relevant to oncology together with basics and advanced training of medical oncology and chemotherapy. The curriculum requires a professional approach from the students who will be expected to have a deep understanding of the subjects. It is expected that the trainees will read the texts and make critical use, where appropriate of original literature and peer scrutinized review articles in the related scientific and clinical literature such that they can aspire to an excellent standard in the practice of medical oncology. Learning methods include interdisciplinary meetings, hospital rounds and outpatient departments with medical oncologists, seminars and workshops, journal club, textbooks, and clerkships.
Especially, this course aims to learn evidence-based cancer chemotherapy and palliative medicine, together with clinical pharmacology, clinical trials, clinical safety, and medical ethics. Students will learn clinical pharmacology and pharmacogenetics of anticancer agents by investigating interindividual variations in drug response and toxicity to cancer chemotherapy and by conducting some clinical trials of cancer chemotherapy.