Laboratories
- Back
- Top > Laboratories > Microbiology and Immunology > Immunology
Microbiology and ImmunologyImmunology
Introduction
The immune system discriminates between self and non-self components and eliminates non-self such as bacteria and virus. Recently, it has been shown that the immune system also plays critical roles in a wide variety of biological activities including cancers, metabolism and pregnancy. Hyper- and hypo-activation of the immune responses, however, induce allergy and autoimmune diseases and chronic infection and cancers, respectively. In cancers, while the immune system controls the development of cancer (immune surveillance), cancer cells survive in the hosts by adapting to the immune-competent environment by reducing their immunogenicity and recruiting immune suppressive cells (immune escape). In our department, we aim at clarifying the detailed mechanisms governing immune surveillance and immune escape through investigating immune homeostasis and immune-associated diseases with basic and translational researches. Then, the entire picture of immune system as a dynamic biological activity would be illuminated.
Research Projects
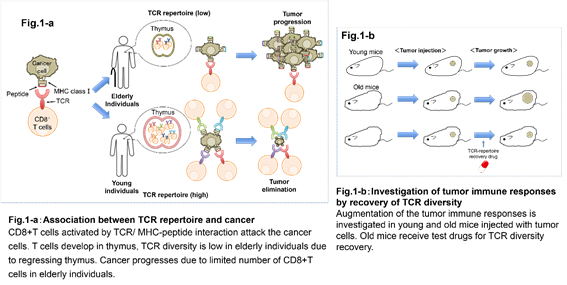
1. New cancer immunotherapy approaches focusing on the diversity of T cells.
Actions of various immune cells in tumor environment involve both elimination and survival of cancer cells. In particular, there are cells in the immune system called CD8-positive (CD8 +) T cells that function to eliminate cancer cells. CD8 + T cells are able to selectively attack tumor cells upon proper activation. This involves recognition of the cancer antigen on tumor cells by T cell receptor (TCR) and co-stimulation of its concomitant receptors. On the other hand, tumors create an immunosuppressive environment employing immune checkpoint molecules signaling (such as CTLA-4 and PD1), immune suppressor cells (such as regulatory T cells and tumor associated macrophages), and various cytokines, concerted actions of which result in the tumor's escape from being attacked by the CD8 + T cells. It is believed that the attenuation of immune responses is one of the cancer’s immunosurveillance escape mechanisms. Another factor affecting immune responses could be low diversity of the repertoires of the tumor infiltrating CD8+ T cells. Each CD8+ T cell expresses a single type of TCR. Thus the diversity of CD8+ T cells is determined by the variety of the TCRs. Because each TCR recognizes only a specific part (epitope) of an antigen, the wider the diversity of TCRs, the more epitopes they could target. Therefore, CD8+ T cells with wider TCR repertoires have higher chances to respond to a particular antigen. We have been focusing on the development of new approaches in cancer immunotherapy based on the diversity of CD8+ T cell repertoires. One of our research targets is the process for the generation of TCR diversity in thymus where T cells develop. Studying low diversity TCR repertoires in aging mice is one of the experimental approaches. We are searching for new molecules that control the variability of TCRs and thus could finally affect the numbers of specific CD8+ T cells capable of attacking a tumor. By comparing them to the existing immunotherapy methods, we can assess whether new candidates molecules have therapeutical advantages. Such molecules may further become candidate drugs to restore / control TCR repertoires.
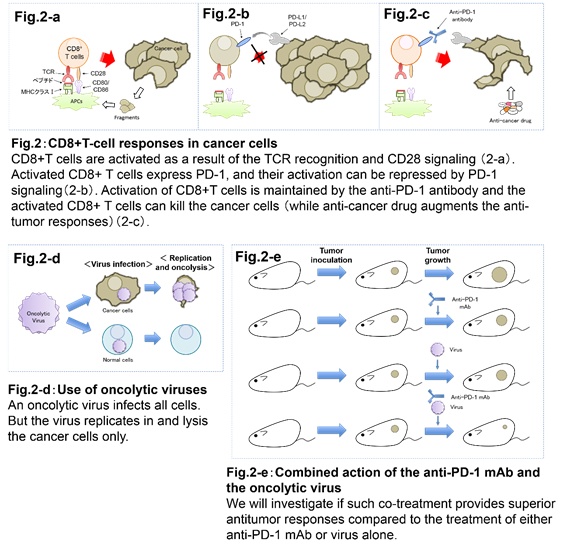
2. Development of new cancer immunotherapy approaches that utilize immune checkpoint inhibitors.
Immune checkpoint inhibitors are expressed when T cell activates. Thus they negatively regulate T cell functions. Normally this system is aimed to suppress autoimmune responses caused by hyperactivation of T cells. On the other hand, tumor environment acts to decrease the host's immune responses by suppressing the activation of T cells using immune checkpoint signaling. Recently, anti-PD-1 antibody has become a central topic in cancer immunotherapy research as it has been shown to enhance the host's immune responses to the tumor by blocking inhibitory PD-1 signaling and thus activating CD8+ T cells. Anti-PD-1 antibody therapy has shown efficacy in refractory cancers. However, due to restrictions in therapeutic effects of the antibody alone, the combination therapy with other drugs seems to be more beneficial. Current clinical trials show that the combination therapy of the antibody with standard regimen anti-cancer drugs has overall survival advantages. We use mouse models in the search for prospective substances which could be effective in the combination therapy. Our research is focused on the development of substances of a viral origin that could be effective in the PD-1 combination therapy.
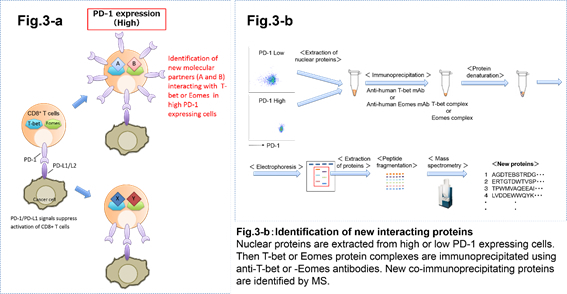
3. Mechanisms of the regulation of transcription factors in immune cells.
Transcription factors regulate expression of genes that control immune cell differentiation, functional properties, migration, etc. For example, FOXP3 is a master regulatory transcription factor in CD4+ regulatory T cells which are immune suppressor cells. T-bet and Eomesodermin (Eomes) are known as master transcription factors which are important in the maintenance of CD8+ T cell functions. Expression of these transcription factors regulates cell's activation and viability. We are analyzing the mechanisms how the expression of these transcription factors is regulated in T cells. Especially in the context of a tumor environment or in response to an antigen stimulation. Using immunoprecipitation and mass spectrometry techniques we are trying to identify new binding partner proteins that interact with and affect functions of these master transcription factors.
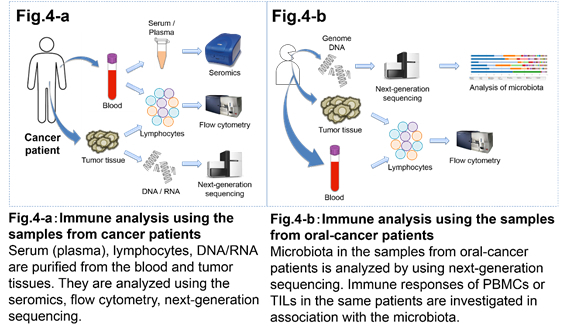
4. Immunotherapy clinical studies involving cancer patients.
It is important to analyze immune responses in cancer patient in order to improve and develop safe and effective immunotherapy regimens. We collaborate with Surgery and Internal medicine departments to analyze tumor tissues and/or blood from cancer patients. In cancer patients the state of immune cells differs between the peripheral blood and the tumor tissue. Thus, comparing immune cells from the peripheral blood and the tumor tissues will allow for new insights in our understanding how the immune response to a tumor develops. We have been collecting samples from cancer patients injected with anti-CTLA-4 and anti-PD-1 antibodies. By comparing the immune responses before and after the antibody therapy, we are aiming to establish the criteria for the effectiveness of the treatment, as well as to establish the causes of autoimmune reactions. In addition, we are analyzing intestinal and oral bacteria to establish new correlations between the microflora and immune responses upon immunotherapy.
5. Associate Professor Suzuki's group’s research work is based on the immunology of CD8+CD122+ cells.
CD122, the b subunit of the receptor for IL-2, is expressed on around 10% of the total murine CD8+ cells. CD4+ cells that express CD25, the a subunit of the receptor for IL-2, are regulatory T cells, which were identified by Dr. Shimon Sakaguchi. In 2004, I (Suzuki) published that CD8+ cells that express CD122 are also regulatory T cells. In 1995, I had established CD122-KO mice, and since then, I have been working on CD122 and CD8+CD122+ cells.
My research theme has been changing gradually compared to that when CD8+CD122+ cells were first discovered, and has been divided and expanded as follows.
i) CD8+CD122+ cells as regulatory T cells
a) We aimed to focus on some special cell population to isolate pure regulatory T cells. We found CD49d, which seemed rather good, but we are still investigating other candidates by using micro-array methods to find a master gene for CD8+ regulatory T cells that corresponds to foxp3 for CD4+Treg, if possible.
b) The main mechanism of regulation is thought to be the induction of cell death in activated T cells using the Fas/FasL pathway whereas other mechanisms, including cytokines such as IL-10, cannot be excluded.
c) Human CD8+ regulatory T cells
Human CD8+ cells appear somewhat different from that of mice. Apart from CD8+ cells, CD8dim cells exist, which must be NK cells because they are TCR-CD122high. Among CD8+ cells, there are no CD122high cells. Therefore, micro-array analysis was performed to compare between CD8+CD122+ cells and CD8+CD122- cells. We selected one gene, CXCR3, and the antibody against human CXCR3 stained 10~20% of CD8+ cells in human peripheral blood. In mice, only Cxcr3 is expressed in the CD8+CD122+ population. These data suggest that CXCR3 can be used for the marker of CD8+ regulatory T cells in humans instead of CD122 in mice.
d) T cells that prevent or suppress immune disorders after bone marrow transplantation
Although transplantation between individuals that have identical genetic background (syngeneic transplantation) is thought to result in no immune response, the real effects are severe dermatitis occasionally or death by pneumonia when mice that lack Fas or FasL (lpr mice or gld mice) are used (our original experimental results, unpublished yet.) These phenomena do not seem to be caused by the response of the immune system against the graft or host tissue but by some insufficient regulation of immune response against exogenous antigens. We plan to expand the experimental system of bone marrow transplantation and clarify which cells work to prevent and suppress dermatitis and pneumonia (CD8+CD122+CD49dlow cells are highly likely). In the experiment of mixed bone marrow transplantation, it is possible to focus on the target cells by selecting wild-type first, CD8KO or CD4KO next, and so on as the partner of mixed bone marrow transplantation for gld and lpr. (In the preliminary experiment so far, the result showed that the target cells were CD8+). The adaptation of cellular therapy using CD8+ regulatory T cells to immunological disorders after bone marrow transplantation, which could be diagnosed as GVHD (graft versus host disease) without any scientific evidence of response of graft versus host, should become more realistic by analyses that show which cells are responsible for the control of effector T cells.
ii) CD8+CD122+ cells as memory cells
Central memory T cells have almost the same cell surface character as CD8+ regulatory T cells. CD8+CD122+CD62L+ (mouse) and CD8+CXCR3+CD45RO+CCR7+ (human) cells are some examples. They (regulatory and memory) could be the same cells, and sometimes they may show regulatory cell activity or sometimes they may behave like memory cells (at least they are not naive cells). Nevertheless, we plan to examine whether CD8+CD122+CD49dlow cells have memory T cell activity.
iii) Relation to tumor immunity
With regards to the adaptation of CD8+CD122+CD49dlow cells to the clinical field, it is important to know the cells that the regulatory cells affect. It is thought that CD4+Treg directly regulates CD4+ cells and the target for the CD8+ regulatory T cells, that is, the CD8+ cells. (CD4+ cells are also regulated by CD8+ regulatory cells but mainly by CD4+ regulatory cells.) CD8+ cytotoxic T cells are importantly known as the final effector in the rejection of transplanted graft or response to cancer cells, and the recent advance of antibody drugs that aim to enhance the immune response against tumors is remarkable. CD8+ regulatory T cells that directly regulate CD8+ cytotoxic effector T cells must be essentially important players of tumor immunity.
Faculty Members
| Faculty | Position | Department |
|---|---|---|
| NISHIKAWA Hiroyoshi | Professor | Immunology |
| AKATSUKA Yoshiki | Designated professor | Immunology |
| ISHIDA Takashi | Designated professor | Immunology |
| SUZUKI Haruhiko | Associate Professor | Immunology |
| HINOHARA Kunihiko | Designated associate professor | Immunology |
| SEO Wooseok | Designated associate professor | Immunology |
| ITO Sachiko | Senior Lecture | Immunology |
| KOCHIN Vitaly | Designated assistant professor | Immunology |
| SUGIYAMA Daisuke | Assistant Professor | Immunology |
| KATO Shinichiro | Designated assistant professor | Immunology |
Bibliography
- 2016
- Akane K, Kojima S, Mak TW, Shiku H, Suzuki H. CD8+CD122+CD49dlow regulatory T cells maintain T-cell homeostasis by killing activated T cells via Fas/FasL-mediated cytotoxicity. Proc Natl Acad Sci U S A, 2016;113:2460-2465.
- Haseda F, Imagawa A, Nishikawa H, Mitsui S, Tsutsumi C, Fujisawa R, Sano H, Murase-Mishiba Y, Terasaki J, Sakaguchi S, Hanafusa T. Antibody to CMRF35-Like Molecule 2, CD300e A Novel Biomarker Detected in Patients with Fulminant Type 1 Diabetes. PLoS One, 2016;11:e0160576.
- Hayakawa Y, Kawada M, Nishikawa H, Ochiya T, Saya H, Seimiya H, Yao R, Hayashi M, Kai C, Matsuda A, Naoe T, Ohtsu A, Okazaki T, Saji H, Sata M, Sugimura H, Sugiyama Y, Toi M, Irimura T. Report on the use of non-clinical studies in the regulatory evaluation of oncology drugs. Cancer Sci, 2016;107:189-202.
- Hirohashi Y, Torigoe T, Tsukahara T, Kanaseki T, Kochin V, Sato N. Immune responses to human cancer stem-like cells/cancer-initiating cells. Cancer Sci, 2016;107:12-17.
- Ito N, Kamiguchi K, Nakanishi K, Sokolovskya A, Hirohashi Y, Tamura Y, Murai A, Yamamoto E, Kanaseki T, Tsukahara T, Kochin V, Chiba S, Shimohama S, Sato N, Torigoe T. A novel nuclear DnaJ protein, DNAJC8, can suppress the formation of spinocerebellar ataxia 3 polyglutamine aggregation in a J-domain independent manner. Biochem Biophys Res Commun, 2016;474:626-633.
- Ito S, Tanaka Y, Oshino R, Okado S, Hori M, Isobe KI. GADD34 suppresses lipopolysaccharide-induced sepsis and tissue injury through the regulation of macrophage activation. Cell Death Dis, 2016;7:e2219.
- Kajiwara T, Tanaka T, Kukita K, Kutomi G, Saito K, Okuya K, Takaya A, Kochin V, Kanaseki T, Tsukahara T, Hirohashi Y, Torigoe T, Hirata K, Sato N, Tamura Y. Hypoxia augments MHC class I antigen presentation via facilitation of ERO1-α-mediated oxidative folding in murine tumor cells. Eur J Immunol, 2016.
- Kakihana K, Fujioka Y, Suda W, Najima Y, Kuwata G, Sasajima S, Mimura I, Morita H, Sugiyama D, Nishikawa H, Hattori M, Hino Y, Ikegawa S, Yamamoto K, Toya T, Doki N, Koizumi K, Honda K, Ohashi K. Fecal microbiota transplantation for patients with steroid-resistant/dependent acute graft-versus-host disease of the gut. Blood, 2016.
- Liu L, Ito S, Nishio N, Sun Y, Tanaka Y, Isobe K. GADD34 Promotes Tumor Growth by Inducing Myeloid-derived Suppressor Cells. Anticancer Res, 2016;36:4623-4628.
- Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, Nagase H, Nishimura J, Yamamoto H, Takiguchi S, Tanoue T, Suda W, Morita H, Hattori M, Honda K, Mori M, Doki Y, Sakaguchi S. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med, 2016;22:679-684.
- Sasaki T, Kanaseki T, Shionoya Y, Tokita S, Miyamoto S, Saka E, Kochin V, Takasawa A, Hirohashi Y, Tamura Y, Miyazaki A, Torigoe T, Hiratsuka H, Sato N. Microenvironmental stresses induce HLA-E/Qa-1 surface expression and thereby reduce CD8(+) T-cell recognition of stressed cells. Eur J Immunol, 2016;46:929-940.
- Shimazu Y, Hishizawa M, Hamaguchi M, Nagai Y, Sugino N, Fujii S, Kawahara M, Kadowaki N, Nishikawa H, Sakaguchi S, Takaori-Kondo A. Hypomethylation of the Treg-Specific Demethylated Region in FOXP3 Is a Hallmark of the Regulatory T-cell Subtype in Adult T-cell Leukemia. Cancer Immunol Res, 2016;4:136-145.
- Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol, 2016;28:401-409.
- Tanaka T, Kutomi G, Kajiwara T, Kukita K, Kochin V, Kanaseki T, Tsukahara T, Hirohashi Y, Torigoe T, Okamoto Y, Hirata K, Sato N, Tamura Y. Cancer-associated oxidoreductase ERO1-α drives the production of VEGF via oxidative protein folding and regulating the mRNA level. Br J Cancer, 2016;114:1227-1234.
- Tanaka Y, Ito S, Isobe K. Vancomycin-sensitive bacteria trigger development of colitis-associated colon cancer by attracting neutrophils. Sci Rep, 2016;6:23920.
- Ureshino H, Shindo T, Nishikawa H, Watanabe N, Watanabe E, Satoh N, Kitaura K, Kitamura H, Doi K, Nagase K, Kimura H, Samukawa M, Kusunoki S, Miyahara M, Shin-I T, Suzuki R, Sakaguchi S, Kimura S. Effector Regulatory T Cells Reflect the Equilibrium between Antitumor Immunity and Autoimmunity in Adult T-cell Leukemia. Cancer Immunol Res, 2016;4:644-649.
- 2015
- Adeegbe DO, Nishikawa H. Regulatory T cells in cancer; can they be controlled? Immunotherapy, 2015;7:843-846.
- Chen N, Nishio N, Ito S, Tanaka Y, Sun Y, Isobe K. Growth arrest and DNA damage-inducible protein (GADD34) enhanced liver inflammation and tumorigenesis in a diethylnitrosamine (DEN)-treated murine model. Cancer Immunol Immunother, 2015;64:777-789.
- Himeno T, Kamiya H, Naruse K, Cheng Z, Ito S, Shibata T, Kondo M, Kato J, Okawa T, Fujiya A, Suzuki H, Kito T, Hamada Y, Oiso Y, Isobe K, Nakamura J. Angioblast Derived from ES Cells Construct Blood Vessels and Ameliorate Diabetic Polyneuropathy in Mice. J Diabetes Res, 2015;2015:257230.
- Hirohashi Y, Torigoe T, Mariya T, Kochin V, Saito T, Sato N. HLA class I as a predictor of clinical prognosis and CTL infiltration as a predictor of chemosensitivity in ovarian cancer. Oncoimmunology, 2015;4:e1005507.
- Ito S, Tanaka Y, Oshino R, Aiba K, Thanasegaran S, Nishio N, Isobe K. GADD34 inhibits activation-induced apoptosis of macrophages through enhancement of autophagy. Sci Rep, 2015;5:8327.
- Kurose K, Ohue Y, Wada H, Iida S, Ishida T, Kojima T, Doi T, Suzuki S, Isobe M, Funakoshi T, Kakimi K, Nishikawa H, Udono H, Oka M, Ueda R, Nakayama E. Phase Ia Study of FoxP3+ CD4 Treg Depletion by Infusion of a Humanized Anti-CCR4 Antibody, KW-0761, in Cancer Patients. Clin Cancer Res, 2015;21:4327-4336.
- Liu L, Ito S, Nishio N, Sun Y, Chen N, Tanaka Y, Isobe K. GADD34 Facilitates Cell Death Resulting from Proteasome Inhibition. Anticancer Res, 2015;35:5317-5324.
- Miyara M, Chader D, Sage E, Sugiyama D, Nishikawa H, Bouvry D, Claër L, Hingorani R, Balderas R, Rohrer J, Warner N, Chapelier A, Valeyre D, Kannagi R, Sakaguchi S, Amoura Z, Gorochov G. Sialyl Lewis x (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans. Proc Natl Acad Sci U S A, 2015;112:7225-7230.
- Nishikawa H. Overview: New Modality for Cancer Treatment. Oncology, 2015;89 Suppl 1:33-35.
- Okabe M, Ito S, Nishio N, Tanaka Y, Isobe K. Thymic Epithelial Cells Induced from Pluripotent Stem Cells by a Three-Dimensional Spheroid Culture System Regenerates Functional T Cells in Nude Mice. Cell Reprogram, 2015;17:368-375.
- Sun Y, Ito S, Nishio N, Tanaka Y, Chen N, Liu L, Isobe K. Enhancement of the acrolein-induced production of reactive oxygen species and lung injury by GADD34. Oxid Med Cell Longev, 2015;2015:170309.
- Takada K, Van Laethem F, Xing Y, Akane K, Suzuki H, Murata S, Tanaka K, Jameson SC, Singer A, Takahama Y. TCR affinity for thymoproteasome-dependent positively selecting peptides conditions antigen responsiveness in CD8(+) T cells. Nat Immunol, 2015;16:1069-1076.
- Tanaka Y, Ito S, Oshino R, Chen N, Nishio N, Isobe K. Effects of growth arrest and DNA damage-inducible protein 34 (GADD34) on inflammation-induced colon cancer in mice. Br J Cancer, 2015;113:669-679.
- Thanasegaran S, Ito S, Nishio N, Uddin MN, Sun Y, Isobe K. Recruitment of Gr1(+)CD11b (+)F4/80 (+) population in the bone marrow and spleen by irradiation-induced pulmonary damage. Inflammation, 2015;38:465-475.
- Torvaldson E, Kochin V, Eriksson JE. Phosphorylation of lamins determine their structural properties and signaling functions. Nucleus, 2015;6:166-171.
- 2014
- de Thonel A, Hazoumé A, Kochin V, Isoniemi K, Jego G, Fourmaux E, Hammann A, Mjahed H, Filhol O, Micheau O, Rocchi P, Mezger V, Eriksson JE, Rangnekar VM, Garrido C. Regulation of the proapoptotic functions of prostate apoptosis response-4 (Par-4) by casein kinase 2 in prostate cancer cells. Cell Death Dis, 2014;5:e1016.
- Isobe K, Cheng Z, Nishio N, Suganya T, Tanaka Y, Ito S. iPSCs, aging and age-related diseases. N Biotechnol, 2014;31:411-421.
- Ito T, Yamada S, Tanaka C, Ito S, Murai T, Kobayashi D, Fujii T, Nakayama G, Sugimoto H, Koike M, Nomoto S, Fujiwara M, Kodera Y. Overexpression of L1CAM is associated with tumor progression and prognosis via ERK signaling in gastric cancer. Ann Surg Oncol, 2014;21:560-568.
- Ito Y, Hashimoto M, Hirota K, Ohkura N, Morikawa H, Nishikawa H, Tanaka A, Furu M, Ito H, Fujii T, Nomura T, Yamazaki S, Morita A, Vignali DA, Kappler JW, Matsuda S, Mimori T, Sakaguchi N, Sakaguchi S. Detection of T cell responses to a ubiquitous cellular protein in autoimmune disease. Science, 2014;346:363-368.
- Kochin V, Shimi T, Torvaldson E, Adam SA, Goldman A, Pack CG, Melo-Cardenas J, Imanishi SY, Goldman RD, Eriksson JE. Interphase phosphorylation of lamin A. J Cell Sci, 2014;127:2683-2696.
- Liu L, Nishio N, Ito S, Tanaka Y, Isobe K. Negative regulation of GADD34 on myofibroblasts during cutaneous wound healing. Biomed Res Int, 2014;2014:137049.
- Maeda Y, Nishikawa H, Sugiyama D, Ha D, Hamaguchi M, Saito T, Nishioka M, Wing JB, Adeegbe D, Katayama I, Sakaguchi S. Detection of self-reactive CD8⁺ T cells with an anergic phenotype in healthy individuals. Science, 2014;346:1536-1540.
- Matsumoto M, Baba A, Yokota T, Nishikawa H, Ohkawa Y, Kayama H, Kallies A, Nutt SL, Sakaguchi S, Takeda K, Kurosaki T, Baba Y. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity, 2014;41:1040-1051.
- Morishima A, Hirano T, Nishikawa H, Nakai K, Sakaguchi S, Kumanogoh A. Comprehensive exploration of autoantibody in Behçet's disease: a novel autoantibody to claudin-1, an essential protein for tight junctions, is identified. Joint Bone Spine, 2014;81:546-548.
- Morita R, Nishizawa S, Torigoe T, Takahashi A, Tamura Y, Tsukahara T, Kanaseki T, Sokolovskaya A, Kochin V, Kondo T, Hashino S, Asaka M, Hara I, Hirohashi Y, Sato N. Heat shock protein DNAJB8 is a novel target for immunotherapy of colon cancer-initiating cells. Cancer Sci, 2014;105:389-395.
- Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol, 2014;27:1-7.
- Nishio N, Ito S, Isobe K. Loss of GADD34 induces early age-dependent deviation to the myeloid lineage. Immunol Cell Biol, 2014;92:170-180.
- Saito T, Wada H, Yamasaki M, Miyata H, Nishikawa H, Sato E, Kageyama S, Shiku H, Mori M, Doki Y. High expression of MAGE-A4 and MHC class I antigens in tumor cells and induction of MAGE-A4 immune responses are prognostic markers of CHP-MAGE-A4 cancer vaccine. Vaccine, 2014;32:5901-5907.
- Sun Y, Ito S, Nishio N, Tanaka Y, Chen N, Isobe K. Acrolein induced both pulmonary inflammation and the death of lung epithelial cells. Toxicol Lett, 2014;229:384-392.
- Tsukahara T, Emori M, Murata K, Hirano T, Muroi N, Kyono M, Toji S, Watanabe K, Torigoe T, Kochin V, Asanuma H, Matsumiya H, Yamashita K, Himi T, Ichimiya S, Wada T, Yamashita T, Hasegawa T, Sato N. Specific targeting of a naturally presented osteosarcoma antigen, papillomavirus binding factor peptide, using an artificial monoclonal antibody. J Biol Chem, 2014;289:22035-22047.
- Wada H, Isobe M, Kakimi K, Mizote Y, Eikawa S, Sato E, Takigawa N, Kiura K, Tsuji K, Iwatsuki K, Yamasaki M, Miyata H, Matsushita H, Udono H, Seto Y, Yamada K, Nishikawa H, Pan L, Venhaus R, Oka M, Doki Y, Nakayama E. Vaccination with NY-ESO-1 overlapping peptides mixed with Picibanil OK-432 and montanide ISA-51 in patients with cancers expressing the NY-ESO-1 antigen. J Immunother, 2014;37:84-92.
- 2013
- Adeegbe DO, Nishikawa H. Natural and induced T regulatory cells in cancer. Front Immunol, 2013;4:190.
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature, 2013;500:232-236.
- Cheng Z, Ito S, Nishio N, Thanasegaran S, Fang H, Isobe K. Characteristics of cardiac aging in C57BL/6 mice. Exp Gerontol, 2013;48:341-348.
- Eikawa S, Kakimi K, Isobe M, Kuzushima K, Luescher I, Ohue Y, Ikeuchi K, Uenaka A, Nishikawa H, Udono H, Oka M, Nakayama E. Induction of CD8 T-cell responses restricted to multiple HLA class I alleles in a cancer patient by immunization with a 20-mer NY-ESO-1f (NY-ESO-1 91-110) peptide. Int J Cancer, 2013;132:345-354.
- Emori M, Tsukahara T, Murase M, Kano M, Murata K, Takahashi A, Kubo T, Asanuma H, Yasuda K, Kochin V, Kaya M, Nagoya S, Nishio J, Iwasaki H, Sonoda T, Hasegawa T, Torigoe T, Wada T, Yamashita T, Sato N. High expression of CD109 antigen regulates the phenotype of cancer stem-like cells/cancer-initiating cells in the novel epithelioid sarcoma cell line ESX and is related to poor prognosis of soft tissue sarcoma. PLoS One, 2013;8:e84187.
- Fujiwara S, Wada H, Kawada J, Kawabata R, Takahashi T, Fujita J, Hirao T, Shibata K, Makari Y, Iijima S, Nishikawa H, Jungbluth AA, Nakamura Y, Kurokawa Y, Yamasaki M, Miyata H, Nakajima K, Takiguchi S, Nakayama E, Mori M, Doki Y. NY-ESO-1 antibody as a novel tumour marker of gastric cancer. Br J Cancer, 2013;108:1119-1125.
- Gupta A, Nuber N, Esslinger C, Wittenbrink M, Treder M, Landshammer A, Noguchi T, Kelly M, Gnjatic S, Ritter E, von Boehmer L, Nishikawa H, Shiku H, Old L, Ritter G, Knuth A, van den Broek M. A novel human-derived antibody against NY-ESO-1 improves the efficacy of chemotherapy. Cancer Immun, 2013;13:3.
- Himeno T, Kamiya H, Naruse K, Cheng Z, Ito S, Kondo M, Okawa T, Fujiya A, Kato J, Suzuki H, Kito T, Hamada Y, Oiso Y, Isobe K, Nakamura J. Mesenchymal stem cell-like cells derived from mouse induced pluripotent stem cells ameliorate diabetic polyneuropathy in mice. Biomed Res Int, 2013;2013:259187.
- Hirayama M, Nishikawa H, Nagata Y, Tsuji T, Kato T, Kageyama S, Ueda S, Sugiyama D, Hori S, Sakaguchi S, Ritter G, Old LJ, Gnjatic S, Shiku H. Overcoming regulatory T-cell suppression by a lyophilized preparation of Streptococcus pyogenes. Eur J Immunol, 2013;43:989-1000.
- Ito S, Tanaka Y, Nishio N, Thanasegaran S, Isobe K. Establishment of self-renewable GM-CSF-dependent immature macrophages in vitro from murine bone marrow. PLoS One, 2013;8:e76943.
- Kito T, Shibata R, Ishii M, Suzuki H, Himeno T, Kataoka Y, Yamamura Y, Yamamoto T, Nishio N, Ito S, Numaguchi Y, Tanigawa T, Yamashita JK, Ouchi N, Honda H, Isobe K, Murohara T. iPS cell sheets created by a novel magnetite tissue engineering method for reparative angiogenesis. Sci Rep, 2013;3:1418.
- Liu B, Ohishi K, Orito Y, Nakamori Y, Nishikawa H, Ino K, Suzuki K, Matsumoto T, Masuya M, Hamada H, Mineno J, Ono R, Nosaka T, Shiku H, Katayama N. Manipulation of human early T lymphopoiesis by coculture on human bone marrow stromal cells: potential utility for adoptive immunotherapy. Exp Hematol, 2013;41:367-376.e361.
- Muraoka D, Nishikawa H, Noguchi T, Wang L, Harada N, Sato E, Luescher I, Nakayama E, Kato T, Shiku H. Establishment of animal models to analyze the kinetics and distribution of human tumor antigen-specific CD8⁺ T cells. Vaccine, 2013;31:2110-2118.
- Noguchi T, Ritter G, Nishikawa H. Antibody-based therapy in colorectal cancer. Immunotherapy, 2013;5:533-545.
- Okawa T, Kamiya H, Himeno T, Kato J, Seino Y, Fujiya A, Kondo M, Tsunekawa S, Naruse K, Hamada Y, Ozaki N, Cheng Z, Kito T, Suzuki H, Ito S, Oiso Y, Nakamura J, Isobe K. Transplantation of neural crest-like cells derived from induced pluripotent stem cells improves diabetic polyneuropathy in mice. Cell Transplant, 2013;22:1767-1783.
- Okuno Y, Murakoshi A, Negita M, Akane K, Kojima S, Suzuki H. CD8+ CD122+ regulatory T cells contain clonally expanded cells with identical CDR3 sequences of the T-cell receptor β-chain. Immunology, 2013;139:309-317.
- Saijo H, Hirohashi Y, Torigoe T, Kochin V, Takahashi H, Sato N. Cytotoxic T lymphocytes: the future of cancer stem cell eradication? Immunotherapy, 2013;5:549-551.
- Shozib HB, Suzuki H, Iino S, Nakayama S. Acceleration of ileal pacemaker activity in mice lacking interleukin 10. Inflamm Bowel Dis, 2013;19:1577-1585.
- Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y, Karbach J, Jäger E, Sakaguchi S. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A, 2013;110:17945-17950.
- Takahashi A, Hirohashi Y, Torigoe T, Tamura Y, Tsukahara T, Kanaseki T, Kochin V, Saijo H, Kubo T, Nakatsugawa M, Asanuma H, Hasegawa T, Kondo T, Sato N. Ectopically expressed variant form of sperm mitochondria-associated cysteine-rich protein augments tumorigenicity of the stem cell population of lung adenocarcinoma cells. PLoS One, 2013;8:e69095.
- Thanasegaran S, Cheng Z, Ito S, Nishio N, Isobe K. No immunogenicity of IPS cells in syngeneic host studied by in vivo injection and 3D scaffold experiments. Biomed Res Int, 2013;2013:378207.
- Yasuda K, Torigoe T, Morita R, Kuroda T, Takahashi A, Matsuzaki J, Kochin V, Asanuma H, Hasegawa T, Saito T, Hirohashi Y, Sato N. Ovarian cancer stem cells are enriched in side population and aldehyde dehydrogenase bright overlapping population. PLoS One, 2013;8:e68187.
- 2012
- Fujiwara S, Wada H, Miyata H, Kawada J, Kawabata R, Nishikawa H, Gnjatic S, Sedrak C, Sato E, Nakamura Y, Sakakibara M, Kanto T, Shimosegawa E, Hatazawa J, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Nakayama E, Mori M, Doki Y. Clinical trial of the intratumoral administration of labeled DC combined with systemic chemotherapy for esophageal cancer. J Immunother, 2012;35:513-521.
- Hirohashi Y, Torigoe T, Inoda S, Morita R, Kochin V, Sato N. Cytotoxic T lymphocytes: Sniping cancer stem cells. Oncoimmunology, 2012;1:123-125.
- Isobe K, Cheng Z, Ito S, Nishio N. Aging in the mouse and perspectives of rejuvenation through induced pluripotent stem cells (iPSCs). Results Probl Cell Differ, 2012;55:413-427.
- Ito S, Nishio N, Isobe K. Analysis of b-amyloid peptide-binding proteins in microglial cells. The Open geriatric medicine journal, 2012; 5: 1-6.
- Iwami K, Shimato S, Ohno M, Okada H, Nakahara N, Sato Y, Yoshida J, Suzuki S, Nishikawa H, Shiku H, Natsume A, Wakabayashi T. Peptide-pulsed dendritic cell vaccination targeting interleukin-13 receptor α2 chain in recurrent malignant glioma patients with HLA-A*24/A*02 allele. Cytotherapy, 2012;14:733-742.
- Kawada J, Wada H, Isobe M, Gnjatic S, Nishikawa H, Jungbluth AA, Okazaki N, Uenaka A, Nakamura Y, Fujiwara S, Mizuno N, Saika T, Ritter E, Yamasaki M, Miyata H, Ritter G, Murphy R, Venhaus R, Pan L, Old LJ, Doki Y, Nakayama E. Heteroclitic serological response in esophageal and prostate cancer patients after NY-ESO-1 protein vaccination. Int J Cancer, 2012;130:584-592.
- Mori T, Nishizawa S, Hirohashi Y, Torigoe T, Tamura Y, Takahashi A, Kochin V, Fujii R, Kondo T, Greene MI, Hara I, Sato N. Efficiency of G2/M-related tumor-associated antigen-targeting cancer immunotherapy depends on antigen expression in the cancer stem-like population. Exp Mol Pathol, 2012;92:27-32.
- Nakamori Y, Liu B, Ohishi K, Suzuki K, Ino K, Matsumoto T, Masuya M, Nishikawa H, Shiku H, Hamada H, Katayama N. Human bone marrow stromal cells simultaneously support B and T/NK lineage development from human haematopoietic progenitors: a principal role for flt3 ligand in lymphopoiesis. Br J Haematol, 2012;157:674-686.
- Nishikawa H, Maeda Y, Ishida T, Gnjatic S, Sato E, Mori F, Sugiyama D, Ito A, Fukumori Y, Utsunomiya A, Inagaki H, Old LJ, Ueda R, Sakaguchi S. Cancer/testis antigens are novel targets of immunotherapy for adult T-cell leukemia/lymphoma. Blood, 2012;119:3097-3104.
- Noguchi T, Kato T, Wang L, Maeda Y, Ikeda H, Sato E, Knuth A, Gnjatic S, Ritter G, Sakaguchi S, Old LJ, Shiku H, Nishikawa H. Intracellular tumor-associated antigens represent effective targets for passive immunotherapy. Cancer Res, 2012;72:1672-1682.
- Suzuki H, Shibata R, Kito T, Yamamoto T, Ishii M, Nishio N, Ito S, Isobe K, Murohara T. Comparative angiogenic activities of induced pluripotent stem cells derived from young and old mice. PLoS One, 2012;7:e39562.
- Suzuki S, Masaki A, Ishida T, Ito A, Mori F, Sato F, Narita T, Ri M, Kusumoto S, Komatsu H, Fukumori Y, Nishikawa H, Tanaka Y, Niimi A, Inagaki H, Iida S, Ueda R. Tax is a potential molecular target for immunotherapy of adult T-cell leukemia/lymphoma. Cancer Sci, 2012;103:1764-1773.
- Uddin MN, Nishio N, Ito S, Suzuki H, Isobe K. Autophagic activity in thymus and liver during aging. Age (Dordr), 2012;34:75-85.
- 2011
- Cheng Z, Ito S, Nishio N, Xiao H, Zhang R, Suzuki H, Okawa Y, Murohara T, Isobe K. Establishment of induced pluripotent stem cells from aged mice using bone marrow-derived myeloid cells. J Mol Cell Biol, 2011;3:91-98.
- Endharti AT, Okuno Y, Shi Z, Misawa N, Toyokuni S, Ito M, Isobe K, Suzuki H. CD8+CD122+ regulatory T cells (Tregs) and CD4+ Tregs cooperatively prevent and cure CD4+ cell-induced colitis. J Immunol, 2011;186:41-52.
- Hayakawa A, Suzuki H, Kamei Y, Tanuma S, Magae J. Cladribine enhances apoptotic cell death in lung carcinoma cells over-expressing DNase γ. Biol Pharm Bull, 2011;34:1001-1004.
- Inami Y, Yoshikai T, Ito S, Nishio N, Suzuki H, Sakurai H, Isobe K. Differentiation of induced pluripotent stem cells to thymic epithelial cells by phenotype. Immunol Cell Biol, 2011;89:314-321.
- Toda M, Wang L, Ogura S, Torii M, Kurachi M, Kakimi K, Nishikawa H, Matsushima K, Shiku H, Kuribayashi K, Kato T. UV irradiation of immunized mice induces type 1 regulatory T cells that suppress tumor antigen specific cytotoxic T lymphocyte responses. Int J Cancer, 2011;129:1126-1136.
- Uddin MN, Ito S, Nishio N, Suganya T, Isobe K. Gadd34 induces autophagy through the suppression of the mTOR pathway during starvation. Biochem Biophys Res Commun, 2011;407:692-698.
- Zhang R, Ito S, Nishio N, Cheng Z, Suzuki H, Isobe KI. Dextran sulphate sodium increases splenic Gr1(+)CD11b(+) cells which accelerate recovery from colitis following intravenous transplantation. Clin Exp Immunol, 2011;164:417-427.
- Zhang R, Ito S, Nishio N, Cheng Z, Suzuki H, Isobe K. Up-regulation of Gr1+CD11b+ population in spleen of dextran sulfate sodium administered mice works to repair colitis. Inflamm Allergy Drug Targets, 2011;10:39-46.
- 2010
- de Thonel A, Ferraris SE, Pallari HM, Imanishi SY, Kochin V, Hosokawa T, Hisanaga S, Sahlgren C, Eriksson JE. Protein kinase Czeta regulates Cdk5/p25 signaling during myogenesis. Mol Biol Cell, 2010;21:1423-1434.
- Mitsui J, Nishikawa H, Muraoka D, Wang L, Noguchi T, Sato E, Kondo S, Allison JP, Sakaguchi S, Old LJ, Kato T, Shiku H. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin Cancer Res, 2010;16:2781-2791.
- Muraoka D, Kato T, Wang L, Maeda Y, Noguchi T, Harada N, Takeda K, Yagita H, Guillaume P, Luescher I, Old LJ, Shiku H, Nishikawa H. Peptide vaccine induces enhanced tumor growth associated with apoptosis induction in CD8+ T cells. J Immunol, 2010;185:3768-3776.
- 2009
- Kaunisto A, Kochin V, Asaoka T, Mikhailov A, Poukkula M, Meinander A, Eriksson JE. PKC-mediated phosphorylation regulates c-FLIP ubiquitylation and stability. Cell Death Differ, 2009;16:1215-1226.
- 2008
- Nishikawa H, Tsuji T, Jäger E, Briones G, Ritter G, Old LJ, Galán JE, Shiku H, Gnjatic S. Induction of regulatory T cell-resistant helper CD4+ T cells by bacterial vector. Blood, 2008;111:1404-1412.
- Nishikawa H, Kato T, Hirayama M, Orito Y, Sato E, Harada N, Gnjatic S, Old LJ, Shiku H. Regulatory T cell-resistant CD8+ T cells induced by glucocorticoid-induced tumor necrosis factor receptor signaling. Cancer Res, 2008;68:5948-5954.
- 2006
- Kochin V, Imanishi SY, Eriksson JE. Fast track to a phosphoprotein sketch - MALDI-TOF characterization of TLC-based tryptic phosphopeptide maps at femtomolar detection sensitivity. Proteomics, 2006;6:5676-5682.
- Nishikawa H, Sato E, Briones G, Chen LM, Matsuo M, Nagata Y, Ritter G, Jäger E, Nomura H, Kondo S, Tawara I, Kato T, Shiku H, Old LJ, Galán JE, Gnjatic S. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J Clin Invest, 2006;116:1946-1954.
- Nishikawa H, Qian F, Tsuji T, Ritter G, Old LJ, Gnjatic S, Odunsi K. Influence of CD4+CD25+ regulatory T cells on low/high-avidity CD4+ T cells following peptide vaccination. J Immunol, 2006;176:6340-6346.
- 2005
- Nishikawa H, Kato T, Tawara I, Takemitsu T, Saito K, Wang L, Ikarashi Y, Wakasugi H, Nakayama T, Taniguchi M, Kuribayashi K, Old LJ, Shiku H. Accelerated chemically induced tumor development mediated by CD4+CD25+ regulatory T cells in wild-type hosts. Proc Natl Acad Sci U S A, 2005;102:9253-9257.
- Nishikawa H, Jäger E, Ritter G, Old LJ, Gnjatic S. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood, 2005;106:1008-1011.
- Nishikawa H, Kato T, Tawara I, Saito K, Ikeda H, Kuribayashi K, Allen PM, Schreiber RD, Sakaguchi S, Old LJ, Shiku H. Definition of target antigens for naturally occurring CD4(+) CD25(+) regulatory T cells. J Exp Med, 2005;201:681-686.
- Nishikawa H, Kato T, Tawara I, Ikeda H, Kuribayashi K, Allen PM, Schreiber RD, Old LJ, Shiku H. IFN-gamma controls the generation/activation of CD4+ CD25+ regulatory T cells in antitumor immune response. J Immunol, 2005;175:4433-4440.
- 2003
- Nishikawa H, Kato T, Tanida K, Hiasa A, Tawara I, Ikeda H, Ikarashi Y, Wakasugi H, Kronenberg M, Nakayama T, Taniguchi M, Kuribayashi K, Old LJ, Shiku H. CD4+ CD25+ T cells responding to serologically defined autoantigens suppress antitumor immune responses. Proc Natl Acad Sci U S A, 2003;100:10902-10906.
- 2001
- Nishikawa H, Tanida K, Ikeda H, Sakakura M, Miyahara Y, Aota T, Mukai K, Watanabe M, Kuribayashi K, Old LJ, Shiku H. Role of SEREX-defined immunogenic wild-type cellular molecules in the development of tumor-specific immunity. Proc Natl Acad Sci U S A, 2001;98:14571-14576.