Laboratories
- Back
- Top > Laboratories > Cell Science > Integrative Physiology
Cell ScienceIntegrative Physiology
Introduction
Our research focuses on brain circuit mechanisms that regulate homeostatic physiological functions in mammals, particularly shedding light on body temperature and metabolic regulation and autonomic stress responses. The homeostatic regulatory functions are essential to our life, particularly to survive a variety of environmental stressors. By combining classic methodologies (e.g., in vivo physiology, electrophysiology and neuroanatomy) with state-of-the-art technologies (e.g., optogenetics and pharmacogenetics), we are pursuing the core mechanisms and principles in the central circuit systems maintaining homeostasis. We believe that our basic research on the fundamental mechanisms of homeostasis would have big impacts on broad areas of clinical researches.
We also study the cellular and molecular mechanisms by which cells sense and respond to mechanical stressors, such as stretch, pressure and gravity. In this project, we use a single-molecular imaging technique and also contribute to a space biology project.
For detailed research information, please visit our original website:
https://www.med.nagoya-u.ac.jp/physiol2/english.html
Research Projects
1. Central neural circuit mechanism for homeostasis
We have revealed the fundamental mechanisms of the brain neuronal circuitries that control body temperature and metabolism, and that develop fever and stress-induced hyperthermia (for our reviews, see Am. J. Physiol., 2011; Temperature, 2015; Pflügers Arch., 2018; BioEssays, 2018; Nature Rev. Neurosci., 2022; Auton. Neurosci., 2022).
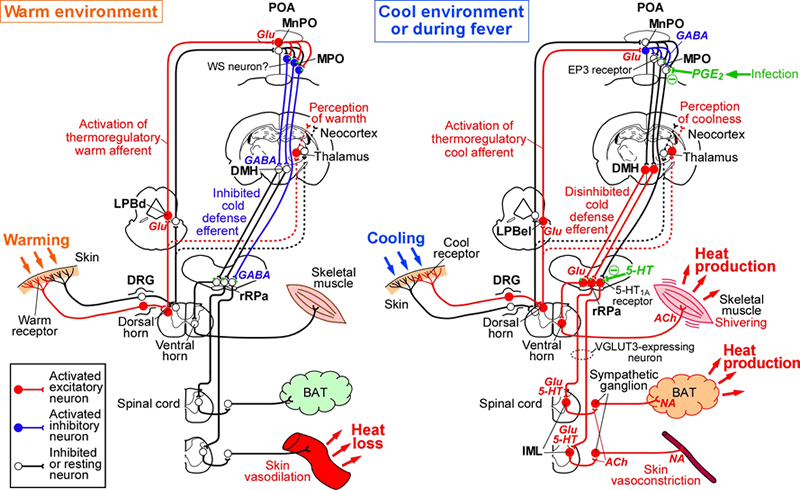
Model of central neural circuitry for body temperature regulation and fever (from K. Nakamura, Am. J. Physiol., 2011)
To further advance our understandings of homeostatic central circuits, we are currently studying the following circuit mechanisms, which are all essential for homeostasis and life. By incorporating state-of-the-art molecular biological technologies into classic physiological and anatomical methodology, we are pursuing the core neural mechanisms and principles in the homeostatic circuits, which have been difficult to approach only with classic techniques.
i) Local circuit mechanism in the control center for body temperature regulation and fever (K. Nakamura et al., J. Neurosci., 2002; J. Neurosci., 2004; Nature Neurosci., 2008; PNAS, 2010; Y. Nakamura et al., Sci. Adv., 2022, etc.)
ii) Neural circuit mechanism for psychological stress-induced hyperthermia (psychogenic fever) and other sympathetic responses (Kataoka et al., Cell Metab., 2014; Science, 2020, etc.)
iii) Neural circuit mechanism that reduces metabolism and promotes feeding behaviors during hunger (Y. Nakamura et al., Cell Metab., 2017, etc.)
iv) Neural circuit mechanism that drives thermoregulatory behaviors to seek for optimal a thermal environment (Yahiro et al., Sci. Rep., 2017, J. Neurosci., 2023, etc.)
v) Central neural pathway for "love hormone" oxytocin to combust fat (Fukushima et al., Cell Rep., 2022, etc.)
vi) Molecular and cellular mechanisms for age-related (middle-age) obesity (Oya et al., Cell Metab., 2024)
More detailed research information is available at our original website.
2. Mechanism of cellular sensing/responding to mechanical stresses
We study the cellular and molecular mechanisms by which cells sense and respond to mechanical stresses, such as stretch, pressure and gravity. In this project, we use a single-molecular imaging technique and also contribute to a space biology project.
Faculty Members
| Faculty | Position | Department |
|---|---|---|
| Kazuhiro Nakamura | Professor | Department of Integrative Physiology |
| Takeshi Kobayashi | Senior Lecturer | Department of Integrative Physiology |
| Yoshiko Nakamura | Senior Lecturer | Department of Integrative Physiology |
| Naoya Kataoka | Designated Senior Lecturer | Department of Integrative Physiology / Institute for Advanced Research |
| Akihiro Fukushima | Designated Senior Lecturer | Department of Integrative Physiology |
| Manami Oya | Designated Senior Lecturer (JST PRESTO Researcher) | Department of Integrative Physiology |
| Yuji Suzuki | Assistant Professor | Department of Integrative Physiology |
| Yuri Kitagawa | Visiting Researcher (JSPS Postdoctoral Research Fellow) | Tokyo Medical and Dental University |
| Misako Takemoto | Technical Staff | Department of Integrative Physiology |
| Hiroko Wakabayashi | Secretary | Department of Integrative Physiology |
Bibliography
- 2025
- Xun Y, Jiang Y, Xu B, Tang M, Ludwig S, Nakamura K, Mukhopadhyay S, Liu C, Beutler B, Zhang Z. GPR45 modulates Gαs at primary cilia of the paraventricular hypothalamus to control food intake. Science, 2025; 388(6751): eadp3989.
- 2024
- Oya M, Miyasaka Y, Nakamura Y, Tanaka M, Suganami T, Mashimo T, Nakamura K*. Age-related ciliopathy: Obesogenic shortening of melanocortin-4 receptor-bearing neuronal primary cilia. Cell Metab, 2024; 36(5): 1044-1058.
- Nakamura K*. Central mechanisms of thermoregulation and fever in mammals. In: Thermal Biology, ed. by Tominaga M, Takagi M: Elsevier, Advances in Experimental Medicine and Biology Vol. 1461, Pages 141-159, 2024.
- 2023
- Yahiro T, Kataoka N, Nakamura K*. Two ascending thermosensory pathways from the lateral parabrachial nucleus that mediate behavioral and autonomous thermoregulation. J Neurosci, 2023; 43: 5221-5240.
- Garami A, Steiner AA, Pakai E, Wanner SP, Almeida MC, Keringer P, Oliveira DL, Nakamura K, Morrison SF, Romanovsky AA. The neural pathway of the hyperthermic response to antagonists of the transient receptor potential vanilloid-1 channel. Temperature, 2023; 10: 121-135.
- Tsuji S, Brace CS, Yao R, Tanie Y, Tada H, Rensing N, Mizuno S, Almunia J, Kong Y, Nakamura K, Furukawa T, Ogiso N, Toyokuni S, Takahashi S, Wong M, Imai SI, Satoh A. Sleep-wake patterns are altered with age, Prdm13 signaling in the DMH, and diet restriction in mice. Life Sci Alliance, 2023; 6: e202301992.
- 2022
- Nakamura Y, Yahiro T, Fukushima A, Kataoka N, Hioki H, Nakamura K*. Prostaglandin EP3 receptor–expressing preoptic neurons bidirectionally control body temperature via tonic GABAergic signaling. Sci Adv, 2022; 8: eadd5463.
- Fukushima A, Kataoka N, Nakamura K*. An oxytocinergic neural pathway that stimulates thermogenic and cardiac sympathetic outflow. Cell Rep, 2022; 40: 111380.
- Nakamura K*, Nakamura Y, Kataoka N. A hypothalamomedullary network for physiological responses to environmental stresses. Nature Rev Neurosci, 2022; 23: 35-52.
- Nakamura K*, Morrison SF. Central sympathetic network for thermoregulatory responses to psychological stress. Auton Neurosci, 2022; 237: 102918.
- Morrison SF, Nakamura K, Tupone D. Thermoregulation in mice: The road to understanding torpor hypothermia and the shortcomings of a circuit for generating fever. Temperature, 2022; 9 (1): 8-11.
- Koba S, Kumada N, Narai E, Kataoka N, Nakamura K, Watanabe T. A brainstem monosynaptic excitatory pathway that drives locomotor activities and sympathetic cardiovascular responses. Nat Commun, 2022; 13: 5079.
- Hayashi Y, Shimizu I, Yoshida Y, Ikegami R, Suda M, Katsuumi G, Fujiki S, Ozaki K, Abe M, Sakimura K, Okuda S, Hayano T, Nakamura K, Walsh K, Jespersen NZ, Nielsen S, Scheele C, Minamino T. Coagulation factors promote brown adipose tissue dysfunction and abnormal systemic metabolism in obesity. iScience, 2022; 25 (7): 104547.
- 2021
- Yoneshiro T, Kataoka N, Walejko JM, Ikeda K, Brown Z, Yoneshiro M, Crown SB, Osawa T, Sakai J, McGarrah RW, White PJ, Nakamura K, Kajimura S. Metabolic flexibility via mitochondrial BCAA carrier SLC25A44 is required for optimal fever. eLife, 2021; 10: e66865.
- Horie T, Nakao T, Miyasaka Y, Nishino T, Matsumura S, Nakazeki F, Ide Y, Kimura M, Tsuji S, Rodriguez RR, Watanabe T, Yamasaki T, Xu S, Otani C, Miyagawa S, Matsushita K, Sowa N, Omori A, Tanaka J, Nishimura C, Nishiga M, Kuwabara Y, Baba O, Watanabe S, Nishi H, Nakashima Y, Picciotto MR, Inoue H, Watanabe D, Nakamura K, Sasaki T, Kimura T, Ono K. microRNA-33 maintains adaptive thermogenesis via enhanced sympathetic nerve activity. Nat Commun, 2021; 12: 843.
- Yoshimi K, Oka Y, Miyasaka Y, Kotani Y, Yasumura M, Uno Y, Hattori K, Tanigawa A, Sato M, Oya M, Nakamura K, Matsushita N, Kobayashi K, Mashimo T. Combi-CRISPR: combination of NHEJ and HDR provides efficient and precise plasmid-based knock-ins in mice and rats. Hum Genet, 2021; 140: 277-287.
- Takahashi M, Ishida Y, Kataoka N, Nakamura K, Hioki H. Efficient labeling of neurons and identification of postsynaptic sites using adeno-associated virus vector. In: Receptor and Ion Channel Detection in the Brain (2nd Ed)., ed. by Lujan R, Ciruela F: Humana, Neuromethods Vol. 169, Pages 323-341, 2021.
- 2020
- Kataoka N, Shima Y, Nakajima K, Nakamura K.* A central master driver of psychosocial stress responses in the rat. Science, 2020; 367: 1105-1112.
- Kataoka N, Nakamura K. Where mind meets body: a master brain circuit for stress responses. TheScienceBreaker, 2020; doi: 10.25250/thescbr.brk404.
- 2019
- Morrison SF, Nakamura K. Central Mechanisms for Thermoregulation. Annu Rev Physiol, 2019; 81: 285-308.
- Ota W, Nakane Y, Kashio M, Suzuki Y, Nakamura K, Mori Y, Tominaga M, Yoshimura T. Involvement of TRPM2 and TRPM8 in temperature-dependent masking behavior. Sci Rep, 2019; 9: 3706.
- 2018
- Nakamura K*, Nakamura Y. Hunger and satiety signaling: Modeling two hypothalamomedullary pathways for energy homeostasis. BioEssays, 2018; 40: 1700252.
- Nakamura Y, Nakamura K.* Central regulation of brown adipose tissue thermogenesis and energy homeostasis dependent on food availability. Pflügers Arch–Eur J Physiol, 2018; 470: 823-837.
- Nakamura K.* Chapter 16 - Afferent pathways for autonomic and shivering thermoeffectors. In: Handbook of Clinical Neurology (Thermoregulation: From Basic Neuroscience to Clinical Neurology Part I), ed. by Romanovsky, A.A.: Elsevier, Vol. 156, Pages 263–279, 2018.
- Koba S*, Hanai E, Kumada N, Kataoka N, Nakamura K, Watanabe T. Sympathoexcitation by hypothalamic paraventricular nucleus neurons projecting to the rostral ventrolateral medulla. J Physiol, 2018; 596: 4581-4595.
- 2017
- Yahiro T, Kataoka N, Nakamura Y, Nakamura K.* The lateral parabrachial nucleus, but not the thalamus, mediates thermosensory pathways for behavioural thermoregulation. Sci Rep, 2017; 7: 5031.
- Nakamura Y, Yanagawa Y, Morrison SF, Nakamura K.* Medullary reticular neurons mediate neuropeptide Y-induced metabolic inhibition and mastication. Cell Metab, 2017; 25: 322–334.
- 2016
- Sohn J, Okamoto S, Kataoka N, Kaneko T, Nakamura K, Hioki H.* Differential inputs to the perisomatic and distal-dendritic compartments of VIP-positive neurons in layer 2/3 of the mouse barrel cortex. Front Neuroanat, 2016; 10: 124.
- Chiba Y, Yamada T*, Tsukita S, Takahashi K, Munakata Y, Shirai Y, Kodama S, Asai Y, Sugisawa T, Uno K, Sawada S, Imai J, Nakamura K, Katagiri H. Dapagliflozin, a sodium-glucose co-transporter 2 inhibitor, acutely reduces energy expenditure in BAT via neural signals in mice. PLoS One, 2016; 11: e0150756.
- 2015
- Nakamura K.* Neural circuit for psychological stress-induced hyperthermia. Temperature, 2015; 2: 352–361.
- 2014
- Kataoka N, Hioki H, Kaneko T, Nakamura K.* Psychological stress activates a dorsomedial hypothalamus–medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab, 2014; 20: 346–358.
- Lkhagvasuren B, Oka T, Nakamura Y, Hayashi N, Sudo N, Nakamura K.* Distribution of Fos-immunoreactive cells in rat forebrain and midbrain following social defeat stress and diazepam treatment. Neuroscience, 2014; 272: 34–57.
- Hiraoka Y, Matsuoka T, Ohno M, Nakamura K, Saijo S, Matsumura S, Nishi K, Sakamoto J, Chen PM, Inoue K, Fushiki T, Kita T, Kimura T, Nishi E.* Critical roles of nardilysin in the maintenance of body temperature homeostasis. Nature Commun, 2014; 5: 3224.
- 2011
- Nakamura K.* Central circuitries for body temperature regulation and fever. Am J Physiol, 2011; 301: R1207–R1228.
- Lkhagvasuren B, Nakamura Y, Oka T, Sudo N, Nakamura K.* Social defeat stress induces hyperthermia through activation of thermoregulatory sympathetic premotor neurons in the medullary raphe region. Eur J Neurosci, 2011; 34: 1442–1452.
- Nakamura K*, Morrison SF. Central efferent pathways for cold-defensive and febrile shivering. J Physiol, 2011; 589: 3641–3658.
- Morrison SF*, Nakamura, K. Central neural pathways for thermoregulation. Front Biosci, 2011; 16: 74–104.
- Zhang ZH, Yu Y, Wei SG, Nakamura Y, Nakamura K, Felder RB.* EP3 receptors mediate PGE2-induced hypothalamic paraventricular nucleus excitation and sympathetic activation. Am J Physiol Heart Circ Physiol, 2011; 301: H1559–H1569.
- Wu S, Esumi S, Watanabe K, Chen J, Nakamura KC, Nakamura K, Kometani K, Minato N, Yanagawa Y, Akashi K, Sakimura K, Kaneko T, Tamamaki N.* Tangential migration and proliferation of intermediate progenitors of GABAergic neurons in the mouse telencephalon. Development, 2011; 138: 2499–2509.
- 2010
- Nakamura K*, Morrison SF. A Thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci USA, 2010; 107: 8848–8853.
- Garami A, Almeida MC, Nucci TB, Hew-Butler T, Soriano RN, Pakai E, Nakamura K, Morrison SF, Romanovsky AA. The TRPV1 channel in normal thermoregulation: What have we learned from experiments using different tools? In: Vanilloid Receptor TRPV1 in Drug Discovery: Targeting Pain and Other Pathological Disorders, ed. by Gomtsyan A, Faltynek CR: John Wiley & Sons, Hoboken, NJ, USA, 2010; 351–402.
- 2009
- Nakamura Y, Nakamura K*, Morrison SF. Different populations of prostaglandin EP3 receptor-expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience, 2009; 161: 614–620.
- Romanovsky AA*, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev, 2009; 61: 228–261.
- Manczak M, Mao P, Nakamura K, Bebbington C, Park B, Reddy PH.* Neutralization of granulocyte macrophage colony-stimulating factor decreases amyloid beta 1-42 and suppresses microglial activity in a transgenic mouse model of Alzheimer's disease. Hum Mol Genet, 2009; 18: 3876–3893.
- Reddy PH*, Manczak M, Zhao W, Nakamura K, Bebbington C, Yarranton G, Mao P. Granulocyte-macrophage colony-stimulating factor antibody suppresses microglial activity: implications for anti-inflammatory effects in Alzheimer's disease and multiple sclerosis. J Neurochem, 2009; 111: 1514–1528.
- 2008
- Nakamura K*, Morrison SF. Preoptic mechanism for cold-defensive responses to skin cooling. J Physiol, 2008; 586: 2611–2620.
- Nakamura K*, Morrison SF. A thermosensory pathway that controls body temperature. Nature Neurosci, 2008; 11: 62–71.
- Morrison SF*, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol, 2008; 93: 773–797.
- Tsuchiya H, Oka T, Nakamura K, Ichikawa A, Saper CB, Sugimoto Y.* Prostaglandin E2 attenuates preoptic expression of GABA(A) receptors via EP3 receptors. J Biol Chem, 2008; 283: 11064–11071.
- 2007
- Nakamura K*, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol, 2007; 292: R127–R136.
- 2006
- Nakamura K*, Yamashita Y, Tamamaki N, Katoh H, Kaneko T, Negishi M. In vivo function of Rnd2 in the development of neocortical pyramidal neurons. Neurosci Res, 2006; 54: 149–153.
- 2005
- Nakamura Y, Nakamura K*, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci, 2005; 22: 3137–3146.
- Nakamura K*, Matsumura K, Kobayashi S, Kaneko T. Sympathetic premotor neurons mediating thermoregulatory functions. Neurosci Res, 2005; 51: 1–8.
- Wu SX, Goebbels S, Nakamura Ko, Nakamura Ka, Kometani K, Minato N, Kaneko T, Nave KA, Tamamaki N.* Pyramidal neurons of upper cortical layers generated by NEX-positive progenitor cells in the subventricular zone. Proc Natl Acad Sci USA, 2005; 102: 17172–17177.
- 2004
- Nakamura K*, Matsumura K, Hübschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogköi Z, König M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci, 2004; 24: 5370–5380.
- Nakamura K*, Wu SX, Fujiyama F, Okamoto K, Hioki H, Kaneko T. Independent inputs by VGLUT2- and VGLUT3-positive glutamatergic terminals onto rat sympathetic preganglionic neurons. NeuroReport, 2004; 15: 431–436.
- Nakamura K.* Fever-inducing sympathetic neural pathways. J Therm Biol, 2004; 29: 339–344.
- Kopp UC*, Cicha MZ, Nakamura K, Nüsing RM, Smith LA, Hökfelt T. Activation of EP4 receptors contributes to prostaglandin E2 mediated stimulation of renal sensory nerves. Am J Physiol Renal Physiol, 2004; 287: F1269–F1282.
- Hioki H, Fujiyama F, Nakamura K, Wu SX, Matsuda W, Kaneko T.* Chemically specific circuit composed of vesicular glutamate transporter 3- and preprotachykinin B-producing interneurons in the rat neocortex. Cereb. Cortex, 2004; 14: 1266–1275.
- 2003
- Yoshida K, Nakamura K, Matsumura K, Kanosue K, König M, Thiel HJ, Boldogköi Z, Toth I, Roth J, Gerstberger R, Hübschle T.* Neurons of the rat preoptic area and the raphe pallidus nucleus innervating the brown adipose tissue express the prostaglandin E receptor subtype EP3. Eur J Neurosci, 2003; 18: 1848–1860.
- 2002
- Nakamura K*, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci, 2002; 22: 4600–4610.
- Mouihate A, Clerget-Froidevaux MS, Nakamura K, Negishi M, Wallace JL, Pittman QJ.* Suppression of fever at near term is associated with reduced COX-2 protein expression in rat hypothalamus. Am J Physiol Regul Integr Comp Physiol, 2002; 283: R800–R805.
- Ishikawa Y, Katoh H, Nakamura K, Mori K, Negishi M.* Developmental changes in expression of small GTPase RhoG mRNA in the rat brain. Brain Res Mol Brain Res, 2002; 106 145–150.
- 2001
- Nakamura K*, Li YQ, Kaneko T, Katoh H, Negishi M. Prostaglandin EP3 receptor protein in serotonin and catecholamine cell groups: a double immunofluorescence study in the rat brain. Neuroscience, 2001; 103: 763–775.
- 2000
- Nakamura K*, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Negishi M. Immunohistochemical localization of prostaglandin EP3 receptor in the rat nervous system. J Comp Neurol, 2000; 421: 543–569.
- Yamaguchi Y, Katoh H, Yasui H, Aoki J, Nakamura K, Negishi M.* Gα(12) and Gα(13) inhibit Ca(2+)-dependent exocytosis through Rho/Rho-associated kinase-dependent pathway. J Neurochem, 2000; 75: 708–717.
- Hasegawa H, Katoh H, Yamaguchi Y, Nakamura K, Futakawa S, Negishi M.* Different membrane targeting of prostaglandin EP3 receptor isoforms dependent on their carboxy-terminal tail structures. FEBS Lett, 2000; 473: 76–80.
- Negishi M*, Katoh H, Nakamura K, Aoki J, Fujita H. Molecular aspects of functions of prostaglandin E receptors in CNS. Recent Res Devel Endocrinol, 2000; 1: 133–143.
- 1999
- Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Ichikawa A, Negishi M.* Immunocytochemical localization of prostaglandin EP3 receptor in the rat hypothalamus. Neurosci Lett, 1999; 260: 117–120.
- Hasegawa H, Fujita H, Katoh H, Aoki J, Nakamura K, Ichikawa A, Negishi M.* Opposite regulation of transepithelial electrical resistance and paracellular permeability by Rho in Madin-Darby canine kidney cells. J Biol Chem, 1999; 274: 20982–20988.
- Aoki J, Katoh H, Yasui H, Yamaguchi Y, Nakamura K, Hasegawa H, Ichikawa A, Negishi M.* Signal transduction pathway regulating prostaglandin EP3 receptor-induced neurite retraction: requirement for two different tyrosine kinases. Biochem J, 1999; 340: 365–369.
- Satoh S, Chang CS, Katoh H, Hasegawa H, Nakamura K, Aoki J, Fujita H, Ichikawa A, Negishi M.* The key amino acid residue of prostaglandin EP3 receptor for governing G protein association and activation steps. Biochem Biophys Res Commun, 1999; 255: 164–168.
- 1998
- Nakamura K, Katoh H, Ichikawa A, Negishi M.* Inhibition of dopamine release by prostaglandin EP3 receptor via pertussis toxin-sensitive and -insensitive pathways in PC12 cells. J Neurochem, 1998; 71: 646–652.
Research Keywords
Body temperature regulation, Fever, Stress, Obesity, Central nervous system, Neural circuit, Autonomic nervous system, Sympathetic, Brown adipose tissue, Homeostasis, Prostaglandin, Oxytocin, Single-molecule imaging, Mechanoreceptor channel
Courses for graduate students
Our academic mission is to perform studies of highest quality that elucidate principles and core mechanisms of neural circuit systems for homeostasis. Another mission of our laboratory is to foster students who will be able to succeed as world-class physiologists and life scientists. We seek for talented graduate students who aim to develop their careers in our laboratory through a two-year Master course and/or a four-year Ph.D. course, which are a part of the government-funded graduate programs at Nagoya University Graduate School of Medicine.
Applicants for these graduate programs should have a strong background in one or more fields of life science including, but not limited to, physiology, neuroanatomy, biochemistry, molecular biology and pharmacology. Strong candidates should show outstanding dexterity and perseverance in any experiments and should have an ability to discuss and write in fluent English or Japanese.
Before application, please contact at:
Kazuhiro Nakamura, Professor
| kazu@med.nagoya-u.ac.jp |
Department of Integrative Physiology
Nagoya University Graduate School of Medicine
65 Tsurumai-cho, Nagoya 466-8550, Japan
| Tel | +81-52-744-2052 |
|---|---|
| Fax | +81-52-744-2056 |