Laboratories
- Back
- Top > Laboratories > Biological Chemistry > Molecular Biology
Biological ChemistryMolecular Biology
Introduction
Our laboratory conducts integrative research ranging from elucidating the fundamental mechanisms of inheritance of genetic information and gene expression regulation—central to all biological phenomena—to the identification of therapeutic targets and the development of treatment strategies for cancer.
This page highlights our three major research themes:
- Identification of therapeutic targets in cancer
- Elucidation of genome stability maintenance mechanisms contributing to tumorigenesis
- Proposal and molecular dissection of checkpoint mechanisms essential for proper cell proliferation
Research Projects
1. Research on Therapeutic Targets in Cancer
Identification of Therapeutic Targets and Development of Treatment Strategies for Refractory Cancers
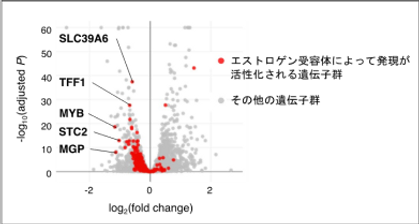
Breast cancer is the most prevalent malignancy among Japanese women, with both incidence and mortality steadily increasing. Approximately 70% of these cases are estrogen receptor alpha (ERα)-positive—tumors expressing ERα, a nuclear receptor for estrogen. Although ERα-targeted endocrine therapies are effective initially, metastatic ERα-positive breast cancers invariably develop resistance over time, posing a significant clinical challenge. To identify novel therapeutic candidates, we applied the following four criteria: (1) physical interaction with ERα, (2) positive correlation of expression levels with ERα, (3) overexpression in breast cancer, and (4) association of high expression with poor patient survival. Using this integrative approach, we identified FKBP52 (FK506 Binding Protein 52) as a prognostic factor and therapeutic candidate (Figure 1) (Habara et al.,PNAS, 2022) 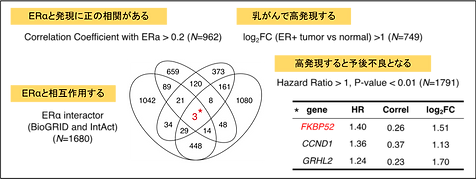
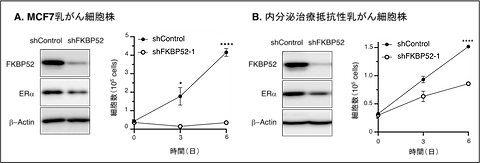
FKBP52 knockdown in MCF‑7 cells resulted in accelerated degradation of ERα, reduced ERα levels, and marked inhibition of cancer cell proliferation.In endocrine therapy–resistant MCF‑7 derivatives (MFR), FKBP52 inhibition similarly decreased ERα expression and suppressed proliferation. (Figure 2)
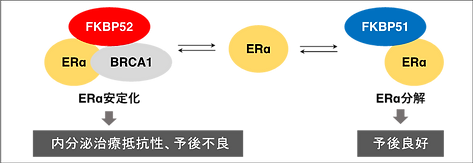
Mechanistically, FKBP52 promotes ERα stabilization by enhancing the ERα–BRCA1 interaction.In contrast, FKBP51, a close homolog of FKBP52, exhibited opposing activity: (a) downregulated in breast cancer, (b) high expression correlated with better survival, and (c) promoted ERα degradation. Collectively, FKBP52 amplifies ERα function and cancer cell proliferation by stabilizing ERα, whereas FKBP51 competes to destabilize ERα (Figure 3)
Calcineurin-Mediated ERα Regulation in Recurrent Breast Cancer
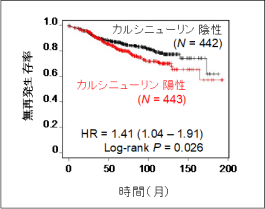
We also identified calcineurin, a calcium‑dependent phosphatasWe have identified calcineurin as a novel regulatory factor involved in breast cancer recurrence, and elucidated the mechanisms by which calcineurin enhances the function of estrogen receptor α (ERα), a key molecule closely associated with the recurrence of malignant tumors. Calcineurin is a crucial Ca2+-dependent phosphatase that mediates calcium signaling and is well known as the molecular target of the immunosuppressive drug FK506, underscoring its clinical importance. While previous studies have suggested that calcineurin promotes cancer cell proliferation, its prognostic significance in cancer patients has remained unclear. In this study, we focused on recurrent breast cancer and demonstrated that high calcineurin expression is correlated with increased recurrence rates and poor prognosis following endocrine therapy (Figure 4).
We have demonstrated that calcineurin enhances the function of estrogen receptor α (ERα) through two distinct mechanisms: (1) by dephosphorylating ERα, calcineurin prevents its degradation; and (2) by facilitating the activation of ERα via mTOR kinase signaling. These dual actions of calcineurin contribute to the increased stability and activity of ERα (Figs. 5 and 6).
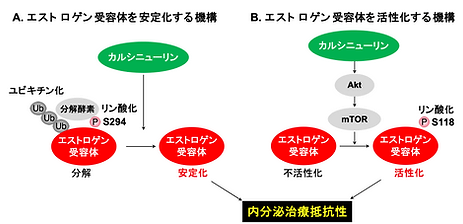
This work advances our understanding of recurrence mechanisms in ERα-positive breast cancer and holds promise for new biomarker discovery and therapeutic development (Masaki & Habara et al., PNAS, 2021)
2. Elucidation of Genome Stability Maintenance Mechanisms Contributing to Tumorigenesis
Regulation of Cell Proliferation and Tumorigenesis via DNA Damage Checkpoints
Using the model organism Schizosaccharomyces pombe (fission yeast), we demonstrated that DNA replication factors are essential for the activation of DNA damage checkpoints (Shimada et al., Mol. Biol. Cell, 1998).Expanding our research to mammalian systems, we further showed that Chk1/Chk2 (Checkpoint kinase 1/2) double knockout mice exhibit enhanced tumorigenesis due to the combined dysfunction of checkpoint signaling and apoptotic pathways (Niida et al., EMBO J., 2010)
Epigenetic Regulation of Tumorigenesis
Mutations in Chk1 induce genomic instability and promote tumor development. Conversely, aberrant Chk1 hyperactivation has been observed in certain malignancies, where it contributes to enhanced proliferation and tumor aggressiveness. We uncovered a previously unrecognized oncogenic function of Chk1: transcriptional activation of proliferation-associated genes via histone phosphorylation (Shimada et al., Cell, 2008 ; Shimada et al., Cell Cycle, 2008). We also discovered that the protein phosphatase PP1 is activated following DNA damage, leading to repression of transcriptional programs associated with cell Proliferation(Shimada et al., EMBO Reports, 2010) (Hanaki et al., Cancer Science, 2021)Furthermore, we identified NIPP1 as a critical regulatory factor for PP1 activation, and elucidated its molecular mechanism of action(Hanaki et al., Cancer Science, 2021). Accurate segregation of chromosomes during mitosis is essential for the faithful transmission of genetic information. Aurora B kinase plays a central role in this process. We identified a novel epigenetic modification—phosphorylation of histone H2AX at serine 121—and demonstrated that this modification is critical for the spatiotemporal regulation of Aurora B kinase activity during mitosis (Shimada et al., Nature Communications, 2016)
3. Proposal and Molecular Elucidation of Checkpoint Mechanisms Essential for Proper Cell Proliferation
Meiotic Recombination Checkpoint Mechanism
Homologous recombination during meiosis is critical both for generating genetic diversity and for ensuring accurate chromosome segregation through the formation of chiasmata. While cell cycle checkpoints have been extensively studied in mitosis, their roles during meiosis remained largely unclear. We proposed the existence of a meiotic recombination checkpoint mechanism that monitors the repair status of programmed DNA double-strand breaks (DSBs) generated during meiotic recombination. Our findings demonstrated that this checkpoint is essential for proper gametogenesis and prevents progression of meiosis in the presence of unrepaired DSBs (Shimada et al., EMBO Journal, 2002).
Checkpoint Mechanism Monitoring Aberrant RNA Metabolism
We also identified a novel RNA surveillance checkpoint mechanism that responds to defects in RNA metabolism. Specifically, we demonstrated that the accumulation of abnormally spliced RNA interferes with mitotic entry by inhibiting the degradation of cyclin B1. This mechanism acts as a quality control system to prevent cell cycle progression under conditions of splicing dysfunction (Shimada et al., Journal of Biological Chemistry, 2005).
Faculty Members
| Faculty | Position | Department |
|---|---|---|
| Midori Shimada | Professor | Molecular Biology |
| Shoma Tsubota | Assistant Professor | Molecular Biology |
| Sakakibara Shotaro | Assistant Professor | Molecular Biology |
| Sugiyama Shigeaki | Assistant Professor | Molecular Biology |
Bibliography
- 2024
- Sato Y, Habara M, Hanaki S, Masaki T, Tomiyasu H, Miki Y, Sakurai M, Morimoto M, Kobayashi D, Miyamoto T, Shimada M*: Calcineurin-mediated dephosphorylation stabilizes E2F1 protein by suppressing binding of the FBXW7 ubiquitin ligase subunit. PNAS, 2024 Oct 8;121(41):e2414618121.
- Sato Y, Habara M, Hanaki S, Sharif J, Tomiyasu H, Miki Y, Shimada M*: Calcineurin/NFATc1 pathway represses cellular cytotoxicity by modulating histone H3 expression. Sci Rep., 14, 14732, 2024.
- Hanaki S, Habara M, Tomiyasu H, Sato Y, Miki Y, Masaki T, Shibutani S, Shimada M*: NFAT activation by FKBP52 promotes cancer cell proliferation by suppressing p53. Life Sci Alliance., 7, e202302426, 2024.
- Tomiyasu H, Habara M, Hanaki S, Sato Y, Miki Y, Shimada M*: FOXO1 promotes cancer cell growth through MDM2-mediated p53 degradation. J. Biol. Chem.,300, 107209, 2024.
- Hanaki S, Habara M, Sato Y, Tomiyasu H, Miki Y, Shibutani S, Shimada M*: Dephosphorylation of NFAT by Calcineurin inhibits. Skp2-mediated degradation. J Biochem., 175, 235-244, 2024.
- 2023
- Masaki T, Habara M, Hanaki S, Sato Y, Tomiyasu H, Miki Y, Shimada M*: Calcineurin-mediated dephosphorylation enhances the stability and transactivation of c-Myc. Sci. Rep., 13, 13116, 2023.
- Masaki T, Habara M, Shibutani S, Hanaki S, Sato Y, Tomiyasu H, Shimada M*: Dephosphorylation of the EGFR protein by calcineurin at serine 1046/1047 enhances its stability. Biochem. Biophys. Res. Commun., 641, 84-92, 2023.
- Suzuki, Y., Kadomatsu, K., Sakamoto, K. Towards the in vivo identification of protein-protein interactions. J Biochem. mvad013 (2023).
- Jun Ouchida, Tomoya Ozaki, Naoki Segi, Yuji Suzuki, Shiro Imagama, Kenji Kadomatsu, Kazuma Sakamoto. Glypican-2 defines age-dependent axonal response to chondroitin sulfate. Exp Neurol. 2023 May 15.doi: 10.1016/j.expneurol.2023.114444.
- 2022
- Habara M, Sato Y, Goshima T, Sakurai M, Imai H, Shimizu H, Katayama Y, Hanaki S, Masaki T, Morimoto M, Nishikawa S, Toyama T, Shimada M*: FKBP52 and FKBP51 Differentially Regulate the Stability of Estrogen Receptor in Breast Cancer. PNAS, 119, e2110256119, 2022.
- Segi, N., Ozaki, T., Suzuki, Y., Ouchida, J., Imagama, S, Kadomatsu, K., Sakamoto, K. Close association of polarization and LC3, a marker of autophagy, in axon determination in mouse hippocampal neurons. Exp Neurol. 114112 (2022). doi: 10.1016/j.expneurol.2022.114112. Online ahead of print.
- 2021
- Masaki T, Habara M, Sato Y, Goshima T, Maeda K, Hanaki S, Shimada M*: Calcineurin regulates the stability and activity of estrogen receptor α. PNAS, 118, e2114258118, 2021.
- Maeda K, Habara M, Kawaguchi M, Matsumoto H, Hanaki S, Masaki T, Sato Y, Matsuyama H, Kunieda K, Nakagawa H, Shimada M*: FKBP51 and FKBP52 regulate androgen receptor dimerization and proliferation in prostate cancer cells. Mol Oncol., 16, 940-956, 2021. [Link]
- Hanaki S, Habara M, Masaki T, Maeda K, Sato Y, Nakanishi M, Shimada M*: PP1 regulatory subunit NIPP1 regulates transcription of E2F1 target genes following DNA damage. Cancer Sci., 112, 2739-2752, 2021.
- Machino M, Gong Y, Ozaki T, Suzuki Y, Watanabe E, Imagama S, Kadomatsu K, Sakamoto K. Dermatan sulfate is an activating ligand of anaplastic lymphoma kinase. J Biochem. 2021 Dec 28;170(5):631-637. doi: 10.1093/jb/mvab085. Online ahead of print. PMID: 34270745
- Gong Y, Abudureyimu S, Kadomatsu K, Sakamoto K. Identification of PTPRσ-interacting proteins by proximity-labelling assay. J Biochem. 2021 Mar 5;169(2):187-194. doi: 10.1093/jb/mvaa141.PMID: 33313879
- Ito S, Ozaki T, Morozumi M, Imagama S, Kadomatsu K, Sakamoto K. Enoxaparin promotes functional recovery after spinal cord injury by antagonizing PTPRσ. Exp. Neurol. 2021 Jun;340:113679. doi: 10.1016/j.expneurol.2021.113679. Epub 2021 Mar 1.
- Sakamoto K, Ozaki T, Suzuki Y, Kadomatsu K. Type IIa RPTPs and Glycans: Roles in Axon Regeneration and Synaptogenesis. Int J Mol Sci. 2021 May 24;22(11):5524. doi:10.3390/ijms22115524.PMID: 34073798
- Sakamoto K, Ozaki T, Kadomatsu K. Axonal Regeneration by Glycosaminoglycan. Front Cell Dev. Biol., 2021 Jun 16;9:702179. doi: 10.3389/fcell.2021.702179. eCollection 2021.
- Komata Y, Tsubota S, Sakamoto K, Ikematsu S, Kadomatsu K. Screening of novel Midkine binding protein by BioID2-based proximity labeling. Nagoya J Med
- 2020
- Mikawa T, Shibata E, Shimada M, Ito K, Ito T, Kanda H, Takubo K, Lleonart ME, Inagaki N, Yokode M, Kondoh H*: Phosphoglycerate mutase cooperates with Chk1 kinase to regulate glycolysis. iScience, 23, 101306, 2020.
- Yamashita K, Kiyonari S, Tsubota S, Kishida S, Sakai R, Kadomatsu K. Thymidylate synthase inhibitor raltitrexed can induce high levels of DNA damage in MYCN-amplified neuroblastoma cells. Cancer Sci. 2020
- 2019
- Nariai Y, Kamino H, Obayashi E, Kato H, Sakashita G, Sugiura T, Migita K, Koga T, Kawakami A, Sakamoto K, Kadomatsu K, Nakakido M, Tsumoto K, Urano T. Generation and characterization of antagonistic anti-human interleukin (IL)-18 monoclonal antibodies with high affinity: Two types of monoclonal antibodies against full-length IL-18 and the neoepitope of inflammatory caspase-cleaved active IL-18. Arch. Biochem. Biophys. 2019 Jan 4;663:71-82.
- Takeda-Okuda N, Mizumoto S, Zhang Z, Kim SK, Lee CH, Jeon BT, Hosaka YZ, Kadomatsu K, Yamada S, Tamura JI. Compositional analysis of the glycosaminoglycan family in velvet antlers of Sika deer (Cervus nippon) at different growing stages. Glycoconj. J. 2019 Jan 24.
- Funahashi Y, Kato N, Masuda T, Nishio F, Kitai H, Ishimoto T, Kosugi T, Tsuboi N, Matsuda N, Maruyama S, Kadomatsu K. miR-146a targeted to splenic macrophages prevents sepsis-induced multiple organ injury. Lab. Invest. 2019 Jan 30.
- Narentuya, Takeda-Uchimura Y, Foyez T, Zhang Z, Akama TO, Yagi H, Kato K, Komatsu Y, Kadomatsu K, Uchimura K. GlcNAc6ST3 is a keratan sulfate sulfotransferase for the protein-tyrosine phosphatase PTPRZ in the adult brain. Sci Rep. 2019 Mar 13;9(1):4387.
- Sakamoto K, Ozaki,T, Yen-Chun Ko, Cheng-Fang Tsai, Gong Y, Morozumi,M, Ishikawa, Y, Uchimura K, Nadanaka S, Kitagawa H, Medel Manuel L. Zulueta, Anandaraju Bandaru, Tamura J, Shang-Cheng Hung, Kadomatsu K. Glycan sulfation patterns define autophagy flux at axon tip via PTPRσ-cortactin axis. Nature Chemical Biology, 2019.
- 2018
- Doke T, Ishimoto T, Hayasaki T, Ikeda S, Hasebe M, Hirayama A, Soga T, Kato N, Kosugi T, Tsuboi N, Lanaspa MA, Johnson RJ, Kadomatsu K, Maruyama S. Lacking Ketohexokinase-A Exacerbates Renal Injury in Streptozotocin-induced Diabetic Mice. Metabolism, 2018.
- Tsubota S, Kadomatsu K. Origin and initiation mechanisms of neuroblastoma. Cell Tissue Res, 2018; 372: 211-221.
- Mori Y, Masuda T, Kosugi T, Yoshioka T, Hori M, Nagaya H, Maeda K, Sato Y, Kojima H, Kato N, Ishimoto T, Katsuno T, Yuzawa Y, Kadomatsu K, Maruyama S. The clinical relevance of plasma CD147/basigin in biopsy-proven kidney diseases. Clin Exp Nephrol, 2018; 22: 815-824.
- 2017
- Su Z, Kishida S, Tsubota S, Sakamoto K, Cao D, Kiyonari S, Ohira M, Kamijo T, Narita A, Xu Y, Takahashi Y, Kadomatsu K. Neurocan, an extracellular chondroitin sulfate proteoglycan, stimulates neuroblastoma cells to promote malignant phenotypes. Oncotarget, 2017; 8: 106296-106310.
- Tsubota S, Kadomatsu K. Origin and mechanism of neuroblastoma. Oncoscience, 2017; 4: 70-72.
- Aynacioglu AS, Bilir A, Kadomatsu K. Dual inhibition of P-glycoprotein and midkine may increase therapeutic effects of anticancer drugs. Med Hypotheses, 2017; 107: 26-28.
- Misa K, Tanino Y, Wang X, Nikaido T, Kikuchi M, Sato Y, Togawa R, Tanino M, Tanaka S, Kadomatsu K, Munakata M. Involvement of midkine in the development of pulmonary fibrosis. Physiol Rep, 2017; 5.
- Tsubota S, Kishida S, Shimamura T, Ohira M, Yamashita S, Cao D, Kiyonari S, Ushijima T, Kadomatsu K. PRC2-Mediated Transcriptomic Alterations at the Embryonic Stage Govern Tumorigenesis and Clinical Outcome in MYCN-Driven Neuroblastoma. Cancer Res, 2017; 77: 5259-5271.
- Takemoto Y, Horiba M, Harada M, Sakamoto K, Takeshita K, Murohara T, Kadomatsu K, Kamiya K. Midkine Promotes Atherosclerotic Plaque Formation Through Its Pro-Inflammatory, Angiogenic and Anti-Apoptotic Functions in Apolipoprotein E-Knockout Mice. Circ J, 2017; 82: 19-27.
- Ichihara-Tanaka K, Kadomatsu K, Kishida S. Temporally and Spatially Regulated Expression of the Linker Histone H1fx During Mouse Development. J Histochem Cytochem, 2017; 65: 513-530.
- Ohgomori T, Yamasaki R, Takeuchi H, Kadomatsu K, Kira JI, Jinno S. Differential activation of neuronal and glial STAT3 in the spinal cord of the SOD1G93A mouse model of amyotrophic lateral sclerosis. Eur J Neurosci, 2017; 46: 2001-2014.
- Tsubota S, Kadomatsu K. Neuroblastoma stem cells and CFC1. Oncotarget, 2017; 8: 45032-45033.
- Mu P, Akashi T, Lu F, Kishida S, Kadomatsu K. A novel nuclear complex of DRR1, F-actin and COMMD1 involved in NF-kappaB degradation and cell growth suppression in neuroblastoma. Oncogene, 2017; 36: 5745-5756.
- Sakamoto K, Kadomatsu K. Mechanisms of axon regeneration: The significance of proteoglycans. Biochim Biophys Acta, 2017; 1861: 2435-2441.
- Ohgomori T, Yamasaki R, Takeuchi H, Kadomatsu K, Kira JI, Jinno S. Differential involvement of vesicular and glial glutamate transporters around spinal alpha-motoneurons in the pathogenesis of SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Neuroscience, 2017; 356: 114-124.
- Jiang W, Ishino Y, Hashimoto H, Keino-Masu K, Masu M, Uchimura K, Kadomatsu K, Yoshimura T, Ikenaka K. Sulfatase 2 Modulates Fate Change from Motor Neurons to Oligodendrocyte Precursor Cells through Coordinated Regulation of Shh Signaling with Sulfatase 1. Dev Neurosci, 2017; 39: 361-374.
- Matsumoto N, Konno A, Ohbayashi Y, Inoue T, Matsumoto A, Uchimura K, Kadomatsu K, Okazaki S. Correction of spherical aberration in multi-focal multiphoton microscopy with spatial light modulator. Opt Express, 2017; 25: 7055-7068.
- Zhang Z, Takeda-Uchimura Y, Foyez T, Ohtake-Niimi S, Narentuya, Akatsu H, Nishitsuji K, Michikawa M, Wyss-Coray T, Kadomatsu K, Uchimura K. Deficiency of a sulfotransferase for sialic acid-modified glycans mitigates Alzheimer's pathology. Proc Natl Acad Sci U S A, 2017; 114: E2947-E2954.
- Yoshimura T, Hayashi A, Handa-Narumi M, Yagi H, Ohno N, Koike T, Yamaguchi Y, Uchimura K, Kadomatsu K, Sedzik J, Kitamura K, Kato K, Trapp BD, Baba H, Ikenaka K. GlcNAc6ST-1 regulates sulfation of N-glycans and myelination in the peripheral nervous system. Sci Rep, 2017; 7: 42257.
- Masuda T, Maeda K, Sato W, Kosugi T, Sato Y, Kojima H, Kato N, Ishimoto T, Tsuboi N, Uchimura K, Yuzawa Y, Maruyama S, Kadomatsu K. Growth Factor Midkine Promotes T-Cell Activation through Nuclear Factor of Activated T Cells Signaling and Th1 Cell Differentiation in Lupus Nephritis. Am J Pathol, 2017; 187: 740-751.
- Hayashi H, Sato W, Kosugi T, Nishimura K, Sugiyama D, Asano N, Ikematsu S, Komori K, Nishiwaki K, Kadomatsu K, Matsuo S, Maruyama S, Yuzawa Y. Efficacy of urinary midkine as a biomarker in patients with acute kidney injury. Clin Exp Nephrol, 2017; 21: 597-607.
- Scilabra SD, Yamamoto K, Pigoni M, Sakamoto K, Muller SA, Papadopoulou A, Lichtenthaler SF, Troeberg L, Nagase H, Kadomatsu K. Dissecting the interaction between tissue inhibitor of metalloproteinases-3 (TIMP-3) and low density lipoprotein receptor-related protein-1 (LRP-1): Development of a \"TRAP\" to increase levels of TIMP-3 in the tissue. Matrix Biol, 2017; 59: 69-79.
- 2016
- Ho WL, Hsu WM, Huang MC, Kadomatsu K, Nakagawara A. Protein glycosylation in cancers and its potential therapeutic applications in neuroblastoma. J Hematol Oncol, 2016; 9: 100.
- Ukai J, Imagama S, Ohgomori T, Ito Z, Ando K, Ishiguro N, Kadomatsu K. Nogo receptor 1 is expressed in both primary cultured glial cells and neurons. Nagoya J Med Sci, 2016; 78: 303-311.
- Zhang Z, Ohtake-Niimi S, Kadomatsu K, Uchimura K. Reduced molecular size and altered disaccharide composition of cerebral chondroitin sulfate upon Alzheimer's pathogenesis in mice. Nagoya J Med Sci, 2016; 78: 293-301.
- Murakami-Tonami Y, Ikeda H, Yamagishi R, Inayoshi M, Inagaki S, Kishida S, Komata Y, Jan K, Takeuchi I, Kondo Y, Maeda T, Sekido Y, Murakami H, Kadomatsu K. SGO1 is involved in the DNA damage response in MYCN-amplified neuroblastoma cells. Sci Rep, 2016; 6: 31615.
- Hayashi H, Sato W, Kosugi T, Nishimura K, Sugiyama D, Asano N, Ikematsu S, Komori K, Nishiwaki K, Kadomatsu K, Matsuo S, Maruyama S, Yuzawa Y. Efficacy of urinary midkine as a biomarker in patients with acute kidney injury. Clin Exp Nephrol, 2016.
- Honda Y, Shishido T, Takahashi T, Watanabe T, Netsu S, Kinoshita D, Narumi T, Kadowaki S, Nishiyama S, Takahashi H, Arimoto T, Miyamoto T, Kishida S, Kadomatsu K, Takeishi Y, Kubota I. Midkine Deteriorates Cardiac Remodeling via Epidermal Growth Factor Receptor Signaling in Chronic Kidney Disease. Hypertension, 2016; 67: 857-865.
- Ohgomori T, Yamada J, Takeuchi H, Kadomatsu K, Jinno S. Comparative morphometric analysis of microglia in the spinal cord of SOD1(G93A) transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci, 2016; 43: 1340-1351.
- Suzuki K, Satoh K, Ikeda S, Sunamura S, Otsuki T, Satoh T, Kikuchi N, Omura J, Kurosawa R, Nogi M, Numano K, Sugimura K, Aoki T, Tatebe S, Miyata S, Mukherjee R, Spinale FG, Kadomatsu K, Shimokawa H. Basigin Promotes Cardiac Fibrosis and Failure in Response to Chronic Pressure Overload in Mice. Arterioscler Thromb Vasc Biol, 2016; 36: 636-646.
- Hashimoto H, Ishino Y, Jiang W, Yoshimura T, Takeda-Uchimura Y, Uchimura K, Kadomatsu K, Ikenaka K. Keratan Sulfate Regulates the Switch from Motor Neuron to Oligodendrocyte Generation During Development of the Mouse Spinal Cord. Neurochem Res, 2016; 41: 450-462.
- 2015
- Kamiguchi H, Kadomatsu K. Introduction to glyco-neuroscience. Exp Neurol, 2015; 274: 89.
- Fujimoto H, Ohgomori T, Abe K, Uchimura K, Kadomatsu K, Jinno S. Time-dependent localization of high- and low-sulfated keratan sulfates in the song nuclei of developing zebra finches. Eur J Neurosci, 2015; 42: 2716-2725.
- Foyez T, Takeda-Uchimura Y, Ishigaki S, Narentuya, Zhang Z, Sobue G, Kadomatsu K, Uchimura K. Microglial keratan sulfate epitope elicits in central nervous tissues of transgenic model mice and patients with amyotrophic lateral sclerosis. Am J Pathol, 2015; 185: 3053-3065.
- Ueno R, Miyamoto K, Tanaka N, Moriguchi K, Kadomatsu K, Kusunoki S. Keratan sulfate exacerbates experimental autoimmune encephalomyelitis. J Neurosci Res, 2015; 93: 1874-1880.
- Takeda-Uchimura Y, Uchimura K, Sugimura T, Yanagawa Y, Kawasaki T, Komatsu Y, Kadomatsu K. Requirement of keratan sulfate proteoglycan phosphacan with a specific sulfation pattern for critical period plasticity in the visual cortex. Exp Neurol, 2015; 274: 145-155.
- Kiyonari S, Iimori M, Matsuoka K, Watanabe S, Morikawa-Ichinose T, Miura D, Niimi S, Saeki H, Tokunaga E, Oki E, Morita M, Kadomatsu K, Maehara Y, Kitao H. The 1,2-Diaminocyclohexane Carrier Ligand in Oxaliplatin Induces p53-Dependent Transcriptional Repression of Factors Involved in Thymidylate Biosynthesis. Mol Cancer Ther, 2015; 14: 2332-2342.
- Maeda K, Kosugi T, Sato W, Kojima H, Sato Y, Kamimura D, Kato N, Tsuboi N, Yuzawa Y, Matsuo S, Murakami M, Maruyama S, Kadomatsu K. CD147/basigin limits lupus nephritis and Th17 cell differentiation in mice by inhibiting the interleukin-6/STAT-3 pathway. Arthritis Rheumatol, 2015; 67: 2185-2195.
- Matsuda Y, Haneda M, Kadomatsu K, Kobayashi T. A proliferation-inducing ligand sustains the proliferation of human naive (CD27(-)) B cells and mediates their differentiation into long-lived plasma cells in vitro via transmembrane activator and calcium modulator and cyclophilin ligand interactor and B-cell mature antigen. Cell Immunol, 2015; 295: 127-136.
- Arima H, Omura T, Hayasaka T, Masaki N, Hanada M, Xu D, Banno T, Kobayashi K, Takeuchi H, Kadomatsu K, Matsuyama Y, Setou M. Reductions of docosahexaenoic acid-containing phosphatidylcholine levels in the anterior horn of an ALS mouse model. Neuroscience, 2015; 297: 127-136.
- Chen D, Ito S, Yuan H, Hyodo T, Kadomatsu K, Hamaguchi M, Senga T. EML4 promotes the loading of NUDC to the spindle for mitotic progression. Cell Cycle, 2015; 14: 1529-1539.
- Ishikawa Y, Imagama S, Ohgomori T, Ishiguro N, Kadomatsu K. A combination of keratan sulfate digestion and rehabilitation promotes anatomical plasticity after rat spinal cord injury. Neurosci Lett, 2015; 593: 13-18.
- Yuan Y, Makita N, Cao D, Mihara K, Kadomatsu K, Takei Y. Atelocollagen-mediated intravenous siRNA delivery specific to tumor tissues orthotopically xenografted in prostates of nude mice and its anticancer effects. Nucleic Acid Ther, 2015; 25: 85-94.
- Lu F, Kishida S, Mu P, Huang P, Cao D, Tsubota S, Kadomatsu K. NeuroD1 promotes neuroblastoma cell growth by inducing the expression of ALK. Cancer Sci, 2015; 106: 390-396.
- Nakaguro M, Kiyonari S, Kishida S, Cao D, Murakami-Tonami Y, Ichikawa H, Takeuchi I, Nakamura S, Kadomatsu K. Nucleolar protein PES1 is a marker of neuroblastoma outcome and is associated with neuroblastoma differentiation. Cancer Sci, 2015; 106: 237-243.
- Sato Y, Sato W, Maruyama S, Wilcox CS, Falck JR, Masuda T, Kosugi T, Kojima H, Maeda K, Furuhashi K, Ando M, Imai E, Matsuo S, Kadomatsu K. Midkine Regulates BP through Cytochrome P450-Derived Eicosanoids. J Am Soc Nephrol, 2015; 26: 1806-1815.
- Kiyonari S, Kadomatsu K. Neuroblastoma models for insights into tumorigenesis and new therapies. Expert Opin Drug Discov, 2015; 10: 53-62.
- Kosugi T, Maeda K, Sato W, Maruyama S, Kadomatsu K. CD147 (EMMPRIN/Basigin) in kidney diseases: from an inflammation and immune system viewpoint. Nephrol Dial Transplant, 2015; 30: 1097-1103.
- 2014
- Satoh K, Satoh T, Kikuchi N, Omura J, Kurosawa R, Suzuki K, Sugimura K, Aoki T, Nochioka K, Tatebe S, Miyamichi-Yamamoto S, Miura M, Shimizu T, Ikeda S, Yaoita N, Fukumoto Y, Minami T, Miyata S, Nakamura K, Ito H, Kadomatsu K, Shimokawa H. Basigin mediates pulmonary hypertension by promoting inflammation and vascular smooth muscle cell proliferation. Circ Res, 2014; 115: 738-750.
- Shinjo R, Imagama S, Ito Z, Ando K, Nishida Y, Ishiguro N, Kadomatsu K. Keratan sulfate expression is associated with activation of a subpopulation of microglia/macrophages in Wallerian degeneration. Neurosci Lett, 2014; 579: 80-85.
- Moreno V, Gonzalo P, Gomez-Escudero J, Pollan A, Acin-Perez R, Breckenridge M, Yanez-Mo M, Barreiro O, Orsenigo F, Kadomatsu K, Chen CS, Enriquez JA, Dejana E, Sanchez-Madrid F, Arroyo AG. An EMMPRIN-gamma-catenin-Nm23 complex drives ATP production and actomyosin contractility at endothelial junctions. J Cell Sci, 2014; 127: 3768-3781.
- Kadomatsu K, Sakamoto K. Mechanisms of axon regeneration and its inhibition: roles of sulfated glycans. Arch Biochem Biophys, 2014; 558: 36-41.
- Miyamoto K, Tanaka N, Moriguchi K, Ueno R, Kadomatsu K, Kitagawa H, Kusunoki S. Chondroitin 6-O-sulfate ameliorates experimental autoimmune encephalomyelitis. Glycobiology, 2014; 24: 469-475.
- Murakami-Tonami Y, Kishida S, Takeuchi I, Katou Y, Maris JM, Ichikawa H, Kondo Y, Sekido Y, Shirahige K, Murakami H, Kadomatsu K. Inactivation of SMC2 shows a synergistic lethal response in MYCN-amplified neuroblastoma cells. Cell Cycle, 2014; 13: 1115-1131.
- Maeda-Hori M, Kosugi T, Kojima H, Sato W, Inaba S, Maeda K, Nagaya H, Sato Y, Ishimoto T, Ozaki T, Tsuboi N, Muro Y, Yuzawa Y, Imai E, Johnson RJ, Matsuo S, Kadomatsu K, Maruyama S. Plasma CD147 reflects histological features in patients with lupus nephritis. Lupus, 2014; 23: 342-352.
- Cao D, Kishida S, Huang P, Mu P, Tsubota S, Mizuno M, Kadomatsu K. A new tumorsphere culture condition restores potentials of self-renewal and metastasis of primary neuroblastoma in a mouse neuroblastoma model. PLoS One, 2014; 9: e86813.
- Muramatsu T, Kadomatsu K. Midkine: an emerging target of drug development for treatment of multiple diseases. Br J Pharmacol, 2014; 171: 811-813.
- Nagaya H, Kosugi T, Maeda-Hori M, Maeda K, Sato Y, Kojima H, Hayashi H, Kato N, Ishimoto T, Sato W, Yuzawa Y, Matsuo S, Kadomatsu K, Maruyama S. CD147/basigin reflects renal dysfunction in patients with acute kidney injury. Clin Exp Nephrol, 2014; 18: 746-754.
- Kadomatsu K, Bencsik P, Gorbe A, Csonka C, Sakamoto K, Kishida S, Ferdinandy P. Therapeutic potential of midkine in cardiovascular disease. Br J Pharmacol, 2014; 171: 936-944.
- Kadomatsu K, Sakamoto K. Sulfated glycans in network rewiring and plasticity after neuronal injuries. Neurosci Res, 2014; 78: 50-54.
- Hoshino H, Foyez T, Ohtake-Niimi S, Takeda-Uchimura Y, Michikawa M, Kadomatsu K, Uchimura K. KSGal6ST is essential for the 6-sulfation of galactose within keratan sulfate in early postnatal brain. J Histochem Cytochem, 2014; 62: 145-156.
- Kishida S, Kadomatsu K. Involvement of midkine in neuroblastoma tumourigenesis. Br J Pharmacol, 2014; 171: 896-904.
- 2013
- Hasan MK, Nafady A, Takatori A, Kishida S, Ohira M, Suenaga Y, Hossain S, Akter J, Ogura A, Nakamura Y, Kadomatsu K, Nakagawara A. ALK is a MYCN target gene and regulates cell migration and invasion in neuroblastoma. Sci Rep, 2013; 3: 3450.
- Matsui H, Ohgomori T, Natori T, Miyamoto K, Kusunoki S, Sakamoto K, Ishiguro N, Imagama S, Kadomatsu K. Keratan sulfate expression in microglia is diminished in the spinal cord in experimental autoimmune neuritis. Cell Death Dis, 2013; 4: e946.
- Duverle DA, Takeuchi I, Murakami-Tonami Y, Kadomatsu K, Tsuda K. Discovering combinatorial interactions in survival data. Bioinformatics, 2013; 29: 3053-3059.
- Hirano K, Ohgomori T, Kobayashi K, Tanaka F, Matsumoto T, Natori T, Matsuyama Y, Uchimura K, Sakamoto K, Takeuchi H, Hirakawa A, Suzumura A, Sobue G, Ishiguro N, Imagama S, Kadomatsu K. Ablation of keratan sulfate accelerates early phase pathogenesis of ALS. PLoS One, 2013; 8: e66969.
- Muramoto A, Imagama S, Natori T, Wakao N, Ando K, Tauchi R, Hirano K, Shinjo R, Matsumoto T, Ishiguro N, Kadomatsu K. Midkine overcomes neurite outgrowth inhibition of chondroitin sulfate proteoglycan without glial activation and promotes functional recovery after spinal cord injury. Neurosci Lett, 2013; 550: 150-155.
- Kadomatsu K, Kishida S, Tsubota S. The heparin-binding growth factor midkine: the biological activities and candidate receptors. J Biochem, 2013; 153: 511-521.
- Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, Kadomatsu K. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis, 2013; 4: e525.
- Kishida S, Mu P, Miyakawa S, Fujiwara M, Abe T, Sakamoto K, Onishi A, Nakamura Y, Kadomatsu K. Midkine promotes neuroblastoma through Notch2 signaling. Cancer Res, 2013; 73: 1318-1327.
- Kojima H, Kosugi T, Sato W, Sato Y, Maeda K, Kato N, Kato K, Inaba S, Ishimoto T, Tsuboi N, Matsuo S, Maruyama S, Yuzawa Y, Kadomatsu K. Deficiency of growth factor midkine exacerbates necrotizing glomerular injuries in progressive glomerulonephritis. Am J Pathol, 2013; 182: 410-419.
- 2012
- Sakamoto K, Kadomatsu K. Midkine in the pathology of cancer, neural disease, and inflammation. Pathol Int, 2012; 62: 445-455.
- Matsumoto T, Imagama S, Hirano K, Ohgomori T, Natori T, Kobayashi K, Muramoto A, Ishiguro N, Kadomatsu K. CD44 expression in astrocytes and microglia is associated with ALS progression in a mouse model. Neurosci Lett, 2012; 520: 115-120.
- Koide N, Yasuda K, Kadomatsu K, Takei Y. Establishment and optimal culture conditions of microrna-induced pluripotent stem cells generated from HEK293 cells via transfection of microrna-302s expression vector. Nagoya J Med Sci, 2012; 74: 157-165.
- Tauchi R, Imagama S, Ohgomori T, Natori T, Shinjo R, Ishiguro N, Kadomatsu K. ADAMTS-13 is produced by glial cells and upregulated after spinal cord injury. Neurosci Lett, 2012; 517: 1-6.
- Tauchi R, Imagama S, Natori T, Ohgomori T, Muramoto A, Shinjo R, Matsuyama Y, Ishiguro N, Kadomatsu K. The endogenous proteoglycan-degrading enzyme ADAMTS-4 promotes functional recovery after spinal cord injury. J Neuroinflammation, 2012; 9: 53.
- Sonobe Y, Li H, Jin S, Kishida S, Kadomatsu K, Takeuchi H, Mizuno T, Suzumura A. Midkine inhibits inducible regulatory T cell differentiation by suppressing the development of tolerogenic dendritic cells. J Immunol, 2012; 188: 2602-2611.
- Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, Hibi H, Kadomatsu K, Ishiguro N, Ueda M. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest, 2012; 122: 80-90.
- Inaba S, Nagahara S, Makita N, Tarumi Y, Ishimoto T, Matsuo S, Kadomatsu K, Takei Y. Atelocollagen-mediated systemic delivery prevents immunostimulatory adverse effects of siRNA in mammals. Mol Ther, 2012; 20: 356-366.
- 2011
- Imagama S, Sakamoto K, Tauchi R, Shinjo R, Ohgomori T, Ito Z, Zhang H, Nishida Y, Asami N, Takeshita S, Sugiura N, Watanabe H, Yamashita T, Ishiguro N, Matsuyama Y, Kadomatsu K. Keratan sulfate restricts neural plasticity after spinal cord injury. J Neurosci, 2011; 31: 17091-17102.
- Huet E, Vallee B, Delbe J, Mourah S, Pruliere-Escabasse V, Tremouilleres M, Kadomatsu K, Doan S, Baudouin C, Menashi S, Gabison EE. EMMPRIN modulates epithelial barrier function through a MMP-mediated occludin cleavage: implications in dry eye disease. Am J Pathol, 2011; 179: 1278-1286.
- Ishiguro H, Horiba M, Takenaka H, Sumida A, Opthof T, Ishiguro YS, Kadomatsu K, Murohara T, Kodama I. A single intracoronary injection of midkine reduces ischemia/reperfusion injury in Swine hearts: a novel therapeutic approach for acute coronary syndrome. Front Physiol, 2011; 2: 27.
- Hayashi M, Kadomatsu K, Kojima T, Ishiguro N. Keratan sulfate and related murine glycosylation can suppress murine cartilage damage in vitro and in vivo. Biochem Biophys Res Commun, 2011; 409: 732-737.
- Kadomatsu K. [Proteoglycans and neural circuit reconstruction]. Seikagaku, 2011; 83: 240-246.
- Kato K, Kosugi T, Sato W, Arata-Kawai H, Ozaki T, Tsuboi N, Ito I, Tawada H, Yuzawa Y, Matsuo S, Kadomatsu K, Maruyama S. Growth factor Midkine is involved in the pathogenesis of renal injury induced by protein overload containing endotoxin. Clin Exp Nephrol, 2011; 15: 346-354.
- Huang P, Kishida S, Cao D, Murakami-Tonami Y, Mu P, Nakaguro M, Koide N, Takeuchi I, Onishi A, Kadomatsu K. The neuronal differentiation factor NeuroD1 downregulates the neuronal repellent factor Slit2 expression and promotes cell motility and tumor formation of neuroblastoma. Cancer Res, 2011; 71: 2938-2948.
- Kato N, Kosugi T, Sato W, Ishimoto T, Kojima H, Sato Y, Sakamoto K, Maruyama S, Yuzawa Y, Matsuo S, Kadomatsu K. Basigin/CD147 promotes renal fibrosis after unilateral ureteral obstruction. Am J Pathol, 2011; 178: 572-579.
- Sakamoto K, Bu G, Chen S, Takei Y, Hibi K, Kodera Y, McCormick LM, Nakao A, Noda M, Muramatsu T, Kadomatsu K. Premature ligand-receptor interaction during biosynthesis limits the production of growth factor midkine and its receptor LDL receptor-related protein 1. J Biol Chem, 2011; 286: 8405-8413.
- Wakao N, Imagama S, Zhang H, Tauchi R, Muramoto A, Natori T, Takeshita S, Ishiguro N, Matsuyama Y, Kadomatsu K. Hyaluronan oligosaccharides promote functional recovery after spinal cord injury in rats. Neurosci Lett, 2011; 488: 299-304.
- 2010
- Hayashi M, Kadomatsu K, Ishiguro N. Keratan sulfate suppresses cartilage damage and ameliorates inflammation in an experimental mice arthritis model. Biochem Biophys Res Commun, 2010; 401: 463-468.
- Ito Z, Sakamoto K, Imagama S, Matsuyama Y, Zhang H, Hirano K, Ando K, Yamashita T, Ishiguro N, Kadomatsu K. N-acetylglucosamine 6-O-sulfotransferase-1-deficient mice show better functional recovery after spinal cord injury. J Neurosci, 2010; 30: 5937-5947.
- Kadomatsu K. Midkine regulation of the renin-angiotensin system. Curr Hypertens Rep, 2010; 12: 74-79.
- Asano Y, Kishida S, Mu P, Sakamoto K, Murohara T, Kadomatsu K. DRR1 is expressed in the developing nervous system and downregulated during neuroblastoma carcinogenesis. Biochem Biophys Res Commun, 2010; 394: 829-835.
- Miwa Y, Yamamoto K, Onishi A, Iwamoto M, Yazaki S, Haneda M, Iwasaki K, Liu D, Ogawa H, Nagasaka T, Uchida K, Nakao A, Kadomatsu K, Kobayashi T. Potential value of human thrombomodulin and DAF expression for coagulation control in pig-to-human xenotransplantation. Xenotransplantation, 2010; 17: 26-37.
- Sumida A, Horiba M, Ishiguro H, Takenaka H, Ueda N, Ooboshi H, Opthof T, Kadomatsu K, Kodama I. Midkine gene transfer after myocardial infarction in rats prevents remodelling and ameliorates cardiac dysfunction. Cardiovasc Res, 2010; 86: 113-121.
- 2009
- Hobo A, Yuzawa Y, Kosugi T, Kato N, Asai N, Sato W, Maruyama S, Ito Y, Kobori H, Ikematsu S, Nishiyama A, Matsuo S, Kadomatsu K. The growth factor midkine regulates the renin-angiotensin system in mice. J Clin Invest, 2009; 119: 1616-1625.
- Kato N, Yuzawa Y, Kosugi T, Hobo A, Sato W, Miwa Y, Sakamoto K, Matsuo S, Kadomatsu K. The E-selectin ligand basigin/CD147 is responsible for neutrophil recruitment in renal ischemia/reperfusion. J Am Soc Nephrol, 2009; 20: 1565-1576.
- Sakakima H, Yoshida Y, Yamazaki Y, Matsuda F, Ikutomo M, Ijiri K, Muramatsu H, Muramatsu T, Kadomatsu K. Disruption of the midkine gene (Mdk) delays degeneration and regeneration in injured peripheral nerve. J Neurosci Res, 2009; 87: 2908-2915.
- Mu P, Nagahara S, Makita N, Tarumi Y, Kadomatsu K, Takei Y. Systemic delivery of siRNA specific to tumor mediated by atelocollagen: combined therapy using siRNA targeting Bcl-xL and cisplatin against prostate cancer. Int J Cancer, 2009; 125: 2978-2990.
- Yin J, Sakamoto K, Zhang H, Ito Z, Imagama S, Kishida S, Natori T, Sawada M, Matsuyama Y, Kadomatsu K. Transforming growth factor-beta1 upregulates keratan sulfate and chondroitin sulfate biosynthesis in microglias after brain injury. Brain Res, 2009; 1263: 10-22.
- Takenaka H, Horiba M, Ishiguro H, Sumida A, Hojo M, Usui A, Akita T, Sakuma S, Ueda Y, Kodama I, Kadomatsu K. Midkine prevents ventricular remodeling and improves long-term survival after myocardial infarction. Am J Physiol Heart Circ Physiol, 2009; 296: H462-469.
- Kobayashi T, Liu D, Ogawa H, Miwa Y, Nagasaka T, Maruyama S, Li YT, Onishi A, Iwamoto M, Kuzuya T, Kadomatsu K, Uchida K, Nakao A. Removal of blood group A/B antigen in organs by ex vivo and in vivo administration of endo-beta-galactosidase (ABase) for ABO-incompatible transplantation. Transpl Immunol, 2009; 20: 132-138.
Research Keywords
Cancer, Genome Stability Maintenance Mechanisms, Cell Cycle
Call for Graduate Students
We welcome the master course and doctor course students