Laboratories
- Back
- Top > Laboratories > Aging Research(Partnership field) > Molecular Aging Research
Aging Research(Partnership field)Molecular Aging Research
Introduction
Department of Aging Research is founded in the Research Institute of the National Center for Geriatrics and Gerontology (https://www.ncgg.go.jp/english/index.html). We focus on the molecular and cellular mechanisms underlying aging and senescence, and promote the cutting-edge researches to profoundly understand and overcome the aging-associated declines and diseases.
Research Projects
- Molecular mechanisms of age-dependent functional decline in immune system and the approach for its recovery
Concerning the functional decline of host defense associated with aging, we are focusing on studying the aging of acquired immune system which is closely related to vaccination and immune therapy using antibodies. Specifically, we aim to be disclosed the molecular mechanisms of functional decline in elderly immune system focusing on aging-related genes with lymphocyte specific expression at the molecular, cellular or even individual level using the animal models. Currently, we are working on the in vitro and in vivo functions of the Zizimin family genes in various immune cells, and finally we hope our study will reach the realization of recovery capable to maintain adequate immunity against infectious diseases that the elderly suffer. - Pathophysiological roles of cellular senescence
Cellular senescence is triggered by sustained and irreparable damage that leads to the activation of tumor suppressor pathways. Increasing amount of evidence suggests that cellular senescence contributes to tissue aging. Senescent cells accumulate in many tissues during aging and are considered to underlie several aging-associated pathologies through their non-cell autonomous function, SASP (senescence-associated secretory phenotype). We thus study the molecular and cellular mechanisms through which senescent cells contribute to tissue aging using model animals.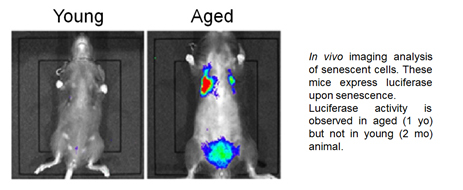
Faculty Members
| Faculty | Position | Department |
|---|---|---|
| Mitsuo Maruyama | Visiting Professor | Aging Research |
Bibliography
- 2016
- Hashimoto M, Asai A, Kawagishi H, Mikawa R, Iwashita Y, Kanayama K, Sugimoto K, Sato T, Maruyama M, Sugimoto M. Elimination of p19ARF-expressing cells enhances pulmonary function in mice. JCI insight, 2016; 1: e88057.
- 2015
- Takagi M, Uno M, Nishii R, Sugimoto M, Hasegawa S, Piao J, Ihara N, Kanai S, Kakei S, Tamura Y, Suganami T, Kamei Y, Shimizu T, Yasuda A, Ogawa Y, Mizutani S. ATM regulates adipocyte differentiation and contributes to glucose homeostasis. Cell Rep., 2015; 10: 957-967.
- Matsuda T, Yanase S, Takaoka A, Maruyama M. The immunosenescence-related gene Zizimin2 is associated with early bone marrow B cell development and marginal zone B cell formation. Immun Ageing., 2015:12: 1.
- 2014
- Hashimoto M, Tsugawa T, Kawagishi H, Asai A, Sugimoto M. Loss of HuR leads to senescence-like cytokine induction in rodent fibroblasts by activating NF-κB. Biochim Biophys Acta., 2014; 1840: 3079-3087.
- Kishimoto M, Matsuda T, Yanase S, Katsumi A, Suzuki N, Ikejiri M, Takagi A, Ikawa M, Kojima T, Kunishima S, Kiyoi H, Naoe T, Matsushita T, Maruyama M. Rhof promotes murine marginal zone B cell development. Nagoya J Med Sci., 2014; 76: 293-305.
- 2013
- Kawagishi H, Hashimoto M, Nakamura H, Tsugawa T, Watanabe A, Kontoyiannis DL, Sugimoto M. HuR maintains replicative lifespan by suppressing ARF tumor suppressor. Mol. Cell. Biol., 2013; 33: 1886-1900.
- Takagi M, Piao J, Kawagichi H, Imai C, Ogawa A, Watanabe A, Akiyama K, Kobayashi C, Mori M, Ko K, Sugimoto M, Mizutani S. Autoimmunity and persistent RAS-mutated clones long after the spontaneous repression of JMML. Leukamia, 2013; 27: 1926-1928.
- Unno J, Takagi M, Piao J, Sugimoto M, Honda F, Maeda D, Masutani M, Kiyono T, Watanabe F, Morio T, Teraoka H, Mizutani S. Artemis-dependent DNA double strand break formation at stalled repilication forks. Cancer Science, 2013; 104: 703-710.
- 2010
- Jia Y, Sakabe I, Matsuda T, Hayakawa T, Maruyama M. Restricted expression of new guanine nucleotide exchange factor Zizimin2 in aged acquired immune system. Nagoya J Med Sci., 2012; 74: 303-311.
- Nakamura H, Kawagishi H, Watanabe A, Sugimoto K, Maruyama M, Sugimoto M. Cooperative role of RNA-binding proteins, Hzf and HuR in p53 activation. Mol. Cell. Biol., 2011; 31: 1997-2009.
- Jia Y, Asai A, Sakabe I, Maruyama M. Rat monoclonal antibodies against new Guanine nucleotide exchange factor, mouse zizimin2. Hybridoma., 2010; 29: 205-209.
- Kawagishi H, Nakamura H, Maruyama M, Mizutani S, Sugimoto K, Takagi M, Sugimoto M. ARF suppresses tumor angiogenesis through translational control of VEGFA mRNA. Cancer Res., 2010; 70: 4749-4758.
- Nakamura H, Azusa A, Maruyama M, Sugimoto M. Production of Rat Monoclonal Antibodies Against RNA-binding Protein, Hzf. Hybridoma, 2010; 29: 7-11.
- 2009
- Wakoh T, Uekawa N, Terauchi K, Sugimoto M, Ishigami A, Shimada J, Maruyama M. Implication of p53-dependent cellular senescence related gene, TARSH in tumor suppression. Biochem. Biophys. Res. Commun., 2009; 380: 807-812.
- Maruyama M, Wakoh T, Terauchi K, Sugimoto M, Shimada J. Putative role of a novel p53-dependent cellular senescence related gene, TARSH in tumor suppression. The Journal of Nutrition, Health and Aging, 2009; 13: S553.
- Wakoh T, Sugimoto M, Terauchi K, Shimada J, Maruyama M. A novel p53-dependent apoptosis function of TARSH in tumor development. Nagoya J. Med. Sci., 2009; 71: 109-114.
- 2008
- Kawagishi H, Wakoh T, Uno H, Maruyama M, Moriya A, Morikawa S, Okano H, Sherr CJ, Takagi M, Sugimoto M. Hzf regulates adipogenesis through translational control of C/EBPalpha. EMBO J.; 2008: 27: 1481-1490.
- 2007
- Uekawa N, Nishioka T, Terauchi K, Ohta S, Sugimoto M, Shimada J, Maruyama M. Generation and characterization of novel monoclonal antibodies against murine and human TARSH proteins. Hybridoma, 2007; 26: 381-386.
- 2005
- Novobrantseva T, Xu S, Tan J.E, Maruyama M, Schwers S, Pelanda R, Lam KP. Stochastic Pairing of Immunoglobulin Heavy and Light Chains Frequently Generates B Cell Antigen Receptors That Are Subject to Editing in Vivo. Int Immunology, 2005; 17: 343-350.
- Uekawa N, Terauchi K, Nishikimi A, Shimada J, Maruyama M. Expression of TARSH gene in MEFs senescence and its potential implication in human lung cancer Biochem Biophys Res Commun., 2005; 329:1031-1038.
- Terauchi K, Shimada J, Uekawa N, Yaoi T, Maruyama M, Fushiki S. Cancer-associated loss of TARSH gene expression in human primary lung cancer. J Cancer Res Clin Oncol., 2005; 4: 1-7.
- Nishikimi A, Meller N, Uekawa N, Isobe K, Schwartz MA, Maruyama M. Zizimin2: a novel, DOCK180-related Cdc42 guanine nucleotide exchange factor expressed predominantly in lymphocytes. FEBS Lett., 2005; 579: 1039-1046.
Research Keywords
Aging, Immunosenescence, Cellular senescence, Tumor suppressor