Laboratories
- Back
- Top > Laboratories > Anatomy and Cell Biology > Cell Biology
Anatomy and Cell BiologyCell Biology
Introduction
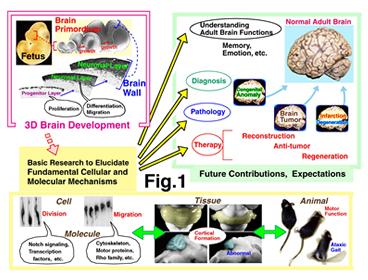
We have been studying how the mammalian brain is constructed (Fig. 1). Structures of the central nervous system including the brain, spinal cord and retina are formed through multiple steps of cellular events. First, neural stem/progenitor cells divide to give rise to neurons. Then, young neurons migrate and find appropriate positions to settle. Finally, post-migratory neurons make connections with other neurons to establish neural circuit. Understanding of the basic mechanisms underlying these brain-forming events is important to elucidate pathological mechanisms of neurological diseases and to develop regenerative treatment methods against brain damages. One of our main strategies is live imaging using fluorescent microscopy. To experimentally ask how cells’ proliferative or migratory behaviors are regulated, we also carry out various experiments by which cell-intrinsic or cell-extrinsic factors are manipulated in culture or in vivo.
Research Projects
1. Systems-biological study to ask how the neuroepithelium is formed and three-dimensionally maintained by efficient assembly of heterogeneously moving cells (Miyata group).
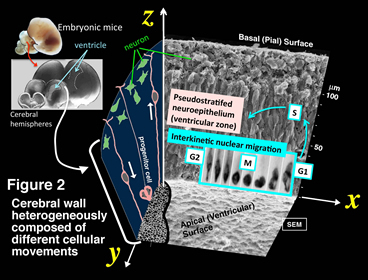
i) Cell biological study to elucidate mechanisms underlying the formation of pseudostratified neuroepithelial structure (Shinoda, Nagasaka, Miyata)
Neural progenitor cells (also called neuroepithelial cells, radial glia, apical progenitors) are highly elongated connecting the apical (ventricular) and the basal (pial) surfaces of developing brain walls (Fig. 2)(Miyata et al., 2001: http://www.sciencedirect.com/science/article/pii/S0896627301004202). They move nuclei/somata in a cell cycle-dependent manner (apically during G2 phase, basally during G1 phase, with cell division at the apical surface). This is called interkinetic nuclear migration (INM) (reviewed in Miyata et al., 2015) (http://journal.frontiersin.org/article/10.3389/fncel.2014.00473/full). This project asks how movements of different cells within the neuroepithelium are coordinated.
ii) Study to elucidate mechanisms underlying the apicobasally elongated morphology of neural progenitor cells (Watanabe, Miyata).
We are asking cell-intrinsic and cell-extrinsic mechanisms that are potentially involved in the elongated morphology of neural progenitor cells.
iii) Measurement and simulation of mechanical properties of neural progenitors (Nagasaka, Shinoda, Miyata)
We are quantitatively measuring mechanical properties of progenitor cells using atomic force microscopy and other methods.
iv) Mechanical factors that neural progenitors sense and respond to (Kawasoe, Saito, Miyata)
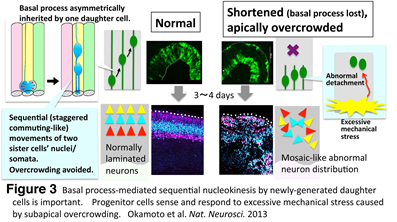
Recently, we found that overcrowded progenitor cells responded to excessive mechanical stress to abnormally detach from the apical surface, which lead to disruption of cortical histogenesis (Fig. 3) (Okamoto et al., Nat. Neurosci. 2013: http://www.nature.com/neuro/journal/v16/n11/full/nn.3525.html). Following this study, we are now investigating mechanism underlying mechanosensation by progenitor cells.
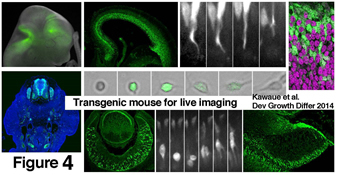
v) Cell-cell interaction in heterogeneously crowded neuroepithelium and its contribution to cell-fate choice (Kawaue, Miyata)
Neuroepithelium is dynamically composed of cells differing in cell cycle phase and differentiation status. We investigate how a daughter cell’s fate is influenced by its interaction with neighboring cells. Three-dimensional imaging using transgenic mouse lines that we recently made (Kawaue et al., Dev. Growth Differ. 2014: http://onlinelibrary.wiley.com/doi/10.1111/dgd.12131/abstract;jsessionid=8FEFD53CB40ED938E616C30E8939A82D.f02t03) will be carried out.
vi) Collective migration of neurons and tissue mechanics (Saito, Miyata).
Mechanical factors should be considered not only for progenitor cells but also for neurons. We are asking this issue using live imaging and mechanical evaluations.
vii) Migration mechanism and role of microglia in embryonic cerebral walls (Hattori, Naito, Miyata)
Although it is known that microglia exist in the developing cerebral cortex from early embryonic days, how they behave there and what factors are involved are unclear. We are investigating this issue by combining neurodevelopmental and immunological approaches.
viii) Intravital live observation of developing brain (Hattori, Saito, Miyata)
Although slice culture methods are powerful to live observe cellular behaviors that construct brains, isolation from the normal (vascularized and complete three-dimensional) environment limits our investigation. Thus, we are trying to perform in utero live observation of developing brain cells.
2. Study to elucidate molecular mechanisms in regulating differential behaviors of neural progenitor cells (Kawaguchi group).
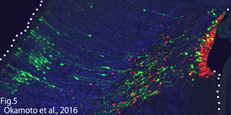
i) Temporal characteristics of the neural progenitor cells (Saito, Kawaguchi)
Cell cycle progression is not necessary for transitions in temporal gene expression and the laminar fate of neural progenitor cells (Okamoto et al., 2016, Fig5). We are investigating “timer” mechanism by which neural progenitor cells know the right developmental time.
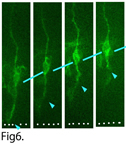
ii) Detachment from the apical surface (Kawaue, Kawaguchi)
Some daughter cells generated by the division of neural progenitor cells detach their apical process from the apical surface and migrate out from the ventricular zone (Fig6). We are studying the molecular mechanisms regulating this detachment.
Faculty Members
| Faculty | Position | Department |
|---|---|---|
| Takaki Miyata | Professor | Anatomy and Cell Biology |
| Yuki Hattori | Associate Professor | Anatomy and Cell Biology |
| Tomoyasu Shinoda | Assistant Professor | Anatomy and Cell Biology |
Bibliography
- 2016
- Pham TQ, Hoshi T, Tanaka Y, Sano A, Kawaue T, Miyata T. Two-Photon imaging of DiO-labelled Meissner corpuscle in living mouse's fingertip. IEEE Trans Haptics, 2016 Jun 1. [epub ahead of print]
- Itoh Y, Higuchi M, Oishi K, Kishi Y, Okazaki T, Sakai H, Miyata T, Nakajima K, Gotoh Y. PDK1–Akt pathway regulates radial neuronal migration and microtubules in the developing mouse neocortex. PNAS, 2016; 113: E2955-E2964.
- Okamoto M, Miyata T, Konno D, Ueda HR, Kasukawa T, Hashimoto M, Matsuzaki F, Kawaguchi A. Cell-cycle-independent transitions in temporal identity of mammalian neural progenitor cells. Nat Commun., 2016; 7:11349.
- Katsunuma S, Honda H, Shinoda T, Ishimoto Y, Miyata T. Kiyonari H, Abe T, Nibu K, Takai Y, Togashi H. Synergistic action of nectins and cadherins generates the mosaic cellular pattern of the olfactory epithelium. J. Cell. Biol., 2016; 212: 561-575.
- Kawaguchi A, Matsuzaki F. Cell cycle–arrested cells know the right time. Cell Cycle, 2016; 15(20): 2683-2684
- 2015
- Leto K, Arancillo M, Becker EBE, Buffo A, Chiang C, Ding B, Dobyns WB, Dusart I, Haldipur P, Hatten ME, Hoshino M, Joyner AL, Kano M, Kilpatrick DL, Koibuchi N, Marion S, Martinez S, Millen K. J, Millner TO, Miyata T, Parmigiani E, Schilling K, Sekerkova G, Sillitoe R. V, Sotelo C, Uesaka N, Wefers A, Wingate RJT, Hawkes R. Consensus paper: Cerebellar development. Cerebellum, 2015 Oct 6. [epub ahead of print]
- Miyata T, Okamoto M, Shinoda T, Kawaguchi A. Interkinetic nuclear migration generates and opposes ventricular-zone crowding: insights into tissue mechanics. Front Cell Neurosci, 2015; 8: 473
- 2014
- Okamoto M, Shinoda T, Kawaue T, Nagasaka A, Miyata T. Ferret-mouse differences in interkinetic nuclear migration and cellular densification in the neocortical ventricular zone. Neurosci. Res, 2014; 83: 25-32
- Kawaue T, Sagou K, Kiyonari H, Ota K, Okamoto M, Shinoda T, Kawaguchi A, Miyata T. Neurogenin2-d4Venus and Gadd45g-d4Venus transgenic mice: Visualizing mitotic and migratory behaviors of cells committed to the neuronal lineage in the developing mammalian brain. Dev Growth Differ, 2014; 56: 293-304
- Hashimoto M, Hata A, Miyata T, Hirase H. Programmable wireless light-emitting diode stimulator for chronic stimulation of optogenetic molecules in freely moving mice. Neurophoton, 2014; 1(1), 011002
- Namba T, Kibe Y, Funahashi Y, Nakamuta S, Takano T, Ueno T, Shimada A, Kozawa S, Okamoto M, Shimoda Y, Oda K, Wada Y, Masuda T, Sakakibara A, Igarashi M, Miyata T, Faivre-Sarrailh C, Takeuchi K, Kaibuchi K. Pioneering axons regulate neuronal polarization in the devveloping cerebral cortex. Neuron, 2014; 81: 814-829
- Sakakibara A (corresponding author), Sato T, Ando R, Noguchi N, Masaoka M, Miyata T. Dynamics of centrosome translocation and microtubule organization in neocortical neurons during distinct modes of polarization. Cereb. Cortex, 2014; 24: 1301-1310
- 2013
- Ageta-Ishihara N, Miyata T, Ohshima C, Watanabe M, Sato Y, Hamamura Y, Higashijima T, Mazitschek R, Bito H, Kinoshita M. Septins promote dendrite and axon development by negatively regulating microtubule stability via HDAC6-mediated deacetylation. Nat Commun, 2013; 4: 2532
- Okamoto M, Namba T, Shinoda T, Kondo T, Watanabe T, Inoue Y, Takeuchi K, Enomoto Y, Ota K, Oda K, Wada Y, Sagou K, Saito K, Sakakibara A, Kawaguchi A, Nakajima K, Adachi T, Fujimori T, Ueda M, Hayashi S, Kaibuchi K, Miyata T. TAG-1–assisted progenitor elongation streamlines nuclear migration to optimize subapical crowding. Nat. Neurosci, 2013; 6: 1556-1566
- Sakakibara A, Ando R, Sapir T, Tanaka T. Microtubule dynamics in neuronal morphogenesis. Open Biol, 2013; 3: 130061
- Sapir T, Levy T, Sakakibara A, Rabinkov A, Miyata T, Reiner O. Shootin1 acts in concert with KIF20B to promote polarization of migrating neurons. J. Neurosci, 2013; 33: 11932-11948
- Wu J, Liu L, Matsuda T, Zao Y, Rebane A, Drobizhev M, Chang Y F, Araki S, Arai Y, March K, Thomas HE, Sagou K, Miyata T, Nagai T, Li WH, Campbell R E. Improved orange and red Ca2+ indicators and photophysical considerations for optogenetic applications. ACS Chem Neurosci, 2013; 4(6): 963-972
- Xie MJ, Yagi H, Kuroda K, Wang CC, Komada M, Zhao H, Sakakibara A, Miyata T, Nagata K, Iguchi T, Sato M. WAVE2-Abi2 complex controls growth cone activity and regulates the multipolar-bipolar transition as well as the initiation of glia-guided migration. Cereb. Cortex, 2013; 23: 1410-1423
- 2012
- Pérez-Martínez FJ, Luque-Río A, Sakakibara A, Hattori M, Miyata T, Luque JM. Reelin-dependent ApoER2 downregulation uncouples newborn neurons from progenitor cells. Biol. Open, 2012; 1: 1258-1263
- 2011
- Nakamuta S, Funahashi Y, Namba T, Arimura N, Picciotto MR, Tokumitsu H, Soderling T R, Sakakibara A, Miyata T, Kamiguchi H, Kaibuchi K. Local application of neurotrophins specifies axons through inositol 1,4,5-trisphosphate, calcium, and ca2+/calmodulin-dependent protein kinases. Sci. Signal, 2011; 4(199): ra76
- Natsume S, Kato T, Kinjo S, Enomoto A, Toda H, Shimato S, Ohka F, Motomura K, Kondo Y, Miyata T, Takahashi M, Wakabayashi T. Girdin maintains the stemness of glioblastoma stem cells. Oncogene, 2011; 31: 2715-2724
- 2010
- Miyata T, Ono Y, Okamoto M, Masaoka M, Sakakibara A, Kawaguchi A, Hashimoto M, Ogawa M. Migration, early axonogenesis, and Reelin-dependent layer-forming behavior of early/posterior-born Purkinje cells in the developing mouse lateral cerebellum. Neural Dev, 2010; 5: 23
- Miyata T, Kawaguchi D, Kawaguchi A, Gotoh Y. Mechanisms that regulate the number of neurons during mouse neocortical development. Curr. Opin. Neurobiol, 2010; 20: 22-28
- Kato TM, Kawaguchi A, Kosodo Y, Niwa H, Matsuzaki F. Lunatic fringe potentiates Notch signaling in the developing brain. Mol Cell Neurosci, 2010; 45: 12-25
- 2009
- Uchida T, Baba A, Perez-Martinez FJ, Hibi T, Miyata T, Luque JM, Nakajima K, Hattori M. Downregulation of functional Reelin receptors in projection neurons implies that primary Reelin action occurs at early/premigratory stages. J Neurosci, 2009; 29: 10653-10662
- Saito K, Dubreuil V, Arai Y, Wilsch-Brauninger M, Schwudke D, Saher G, Miyata T, Breier G, Thiele C, Shevchenko A, Nave KA, Huttner WB. Ablation of cholesterol biosynthesis in neural stem cells increases their VEGF expression and angiogenesis but causes neuron apoptosis. Proc Natl Acad Sci USA, 2009; 106(20): 8350-8355
- Minobe S, Sakakibara A, Ohdachi T, Kanda R, Kimura M, Nakatani S, Tadokoro R, Ochiai W, Nishizawa Y, Mizoguchi A, Kawauchi T, Miyata T. Rac is involved in the interkinetic nuclear migration of cortical progenitor cells. Neurosci. Res, 2009; 63: 294-301
- Ochiai W, Nakatani S, Takahara T, Kainuma M, Masaoka M, Minobe S, Namihira M, Nakashima K, Sakakibara A, Ogawa M, Miyata T. Periventricular Notch activation and asymmetric Ngn2 and Tbr2 expression in pair-generated neocortical daughter cells. Mol. Cell. Neurosci, 2009; 40: 225-233
- 2008
- Yoon KJ, Koo BK, Jeong HW, Ghim J, Kwon MC, Moon JS, Miyata T, Kong Y. Mind bomb 1-experssing intermediate progenitors generate Notch signaling to maintain radial glial cells. Neuron, 2008; 58: 519-531
- Sunabori T, Tokunaga A, Nagai T, Sawamoto K, Okabe M, Miyawaki A, Matsuzaki Y, Miyata T, Okano H. Cell-cycle-specific nestin expression coordinates with morphological changes in embryonic cortical neural progenitors. J Cell Sci, 2008; 121: 1204-1212
- Koyasu T, Kondo M, Miyata K, Ueno S, Miyata T, Nishizawa Y, Terasaki H. Photopic electroretinograms of mGluR6-deficient mice. Curr Eye Res, 2008; 33: 91-99
- Miyata T. Development of three-dimensional architecture of the neuroepithelium: Role of pseudostratification and cellular 'community. Dev. Growth Differ, 2008; 50: S105-S112
- Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, Imamura T, Ogawa M, Masai H, Miyawaki, A. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell, 2008; 132: 487-498
- Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, Matsuzaki F. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat. Cell Biol, 2008; 10: 93-101
- 2007
- Nishizawa Y, Imafuku H, Saito K, Kanda R, Kimura M, Minobe S, Miyazaki F, Kawakatsu S, Masaoka M, Ogawa M, Miyata T. Survey of the morphogenetic dynamics of the ventricular surface of the developing mouse cortex. Dev. Dyn, 2007; 236: 3061-3070
- Tamai H, Shinohara H, Miyata T, Saito K, Nishizawa Y, Nomura T, Osumi N. Pax6 transcription factor regulates interkinetic nuclear movement in cortical progenitor cells via centrosomal stabilization. Genes Cells, 2007; 12: 983-996
- Miyata T. Morphology and mechanics of daughter cells \"delaminating\" from the ventricular zone of the developing neocortex. Cell Adh. Migr, 2007; 1: 99-101
- Miyata T, Ogawa M. Twisting of neocortical progenitor cells underlies a spring-like mechanism for daughter cell migration. Curr, Biol, 2007; 17: 146-151
- Ochiai W, Minobe S, Ogawa M, Miyata T. Transformation of pin-like ventricular zone cells into cortical neurons. Neurosci. Res, 2007; 57: 326-329
- Miyata T. Asymmetric cell division during brain morphogenesis. Prog. Mol. Subcell. Biol, 2007; 452: 121-142
- 2006
- Hirai S, Cui DF, Miyata T, Ogawa M, Kiyonari H, Suda Y, Aizawa S, Banda Y, Ohno S. The c-Jun N-terminal kinase activator dual leucine zipper kinase regulates axon growth and neuronal migration in the developing cerebral cortex. J. Neurosci, 2006; 26: 11992-12002
- Imai F, Hirai S, Akimoto K, Koyama H, Miyata T, Ogawa M, Noguchi S, Sasaoka T, Noda T, Ohno S. Inactivation of aPKCλ results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development, 2006; 133: 1735-1744
- Mutoh T, Miyata T, Kashiwagi S, Miyawaki A, Ogawa M. Dynamic behavior of individual cells in developing organotypic brain slices revealed by the photoconvertable protein Kaede. Exp. Neurol, 2006; 200: 430-437
- Naruse M, Nakahira E, Miyata T, Hitoshi S, Ikenaka K, Bansai R. Induction of oligodendrocyte progenitors in dorsal forebrain by intraventricular microinjection of FGF-2. Dev. Biol, 2006; 60: 1084-1100
- Zou P, Muramatsu H, Miyata T, Muramatsu T. Midkine, a heparin-binding growth factor, is expressed in neural precursor cells and promotes their growth. J. Neurochem, 2006; 99: 1470-1479
- 2005
- Miyata T, Saito K, Nishizawa Y, Murayama A, Masaoka M, and Ogawa M. Modern slice culture for direct observation of production and migration of brain neurons. Nagoya J. Med. Sci. 2005; 67: 65-70
- Ueno S, Kondo M, Miyata K, Hirai T, Miyata T, Usukura J, Nishizawa Y, Miyake Y. Physiological function of S-cone system is not enhanced in rd7 mice. Exp Eye Res, 2005; 81: 751-758
- Uematsu J, Nishizawa Y, Hirako Y, Kitamura K, Usukura J, Miyata T, Owaribe K. Both type-I hemidesmosomes and adherens-type junctions contribute to the cell-substratum adhesion system in myoepithelial cells. Eur J Cell Biol, 2005; 84: 407-415
- 2004
- Kawaguchi A, Ogawa M, Saito K, Matsuzaki F, Okano H, Miyata T. Differential expression of Pax6 and Ngn2 between pair-generatged cortical neurons. J. Neurosci. Res, 2004; 78: 784-795
- Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development, 2004; 131: 3133-3145
Research Keywords
Cerebral cortex、cerebellum、retina、development、neural progenitor cell、neuron、microglia、cell morphology、proliferation、migration、asymmetric cell division、cell-fate choice、slice culture、imaging、mechanics、crowd dynamics