Research in Nakamura Lab at Nagoya University Graduate School of Medicine
We are pursuing the central mechanisms that regulate homeostatic physiological functions in mammals, particularly focusing on body temperature regulation (thermoregulation).
 Human body temperature is maintained at approximately 37°C. The body core temperature elevated or lowered by a few degrees Celcius from the optimal range causes many problems in body functions and can lead to death. So, the body has the systems that prevent elevation of body temperature in hot environments by facilitating sweating and that keep the body warm in cold environments by producing heat through shivering. The systems controlling these homeostatic responses are located in the brain and function independently of our will and consciousness.
Human body temperature is maintained at approximately 37°C. The body core temperature elevated or lowered by a few degrees Celcius from the optimal range causes many problems in body functions and can lead to death. So, the body has the systems that prevent elevation of body temperature in hot environments by facilitating sweating and that keep the body warm in cold environments by producing heat through shivering. The systems controlling these homeostatic responses are located in the brain and function independently of our will and consciousness.
 Fever is a common symptom developed following infection with viruses and bacteria (imagine a cold or flu). This is a host-defense response that reduces the growth of pathogens in the body. When infection occurs, the immune system is activated and communicates to the brain, resulting in the production of the pyrogenic mediator, prostaglandin E2 (PGE2) in the brain. PGE2 then triggers fever by acting on thermoregulatory neurons in the hypothalamus. Antipyretics, such as aspirin and acetoaminophen, exert their effect by blocking the production of PGE2.
Fever is a common symptom developed following infection with viruses and bacteria (imagine a cold or flu). This is a host-defense response that reduces the growth of pathogens in the body. When infection occurs, the immune system is activated and communicates to the brain, resulting in the production of the pyrogenic mediator, prostaglandin E2 (PGE2) in the brain. PGE2 then triggers fever by acting on thermoregulatory neurons in the hypothalamus. Antipyretics, such as aspirin and acetoaminophen, exert their effect by blocking the production of PGE2.
Any disorders in homeostatic functions including body temperature regulation can immediately lead to life-threatening problems. Therefore, research to understand the mechanisms of homeostatic functions is very important not only in basic medicine and biology but also in clinical medicine. This research field has a long history, but a number of important questions still remain to be answered. In particular, the principle of function and the core mechanism of the brain neuronal circuits that maintain homeostasis are still big open questions in medicine and biology. Fortunately, the remarkable progress of biotechnology and combinations of classic methodologies (e.g., in vivo physiology and neuroanatomy) with state-of-the-art technologies (e.g., optogenetics and pharmacogenetics) now allow us to answer such fundamental questions. We believe that our basic research on the fundamental mechanism of homeostasis would have big impacts on broad areas of clinical researches.
Our research findings: Brain mechanisms for homeostasis
We have revealed the fundamental mechanisms of the brain neuronal circuitries that control body temperature and metabolism, and that develop fever and stress-induced hyperthermia (for review, see Nakamura, Am. J. Physiol., 2011; Temperature, 2015; Pflügers Arch., 2018; BioEssays, 2018; Nature Rev. Neurosci., 2022; etc).
1) Thermosensory neural pathways to defend body temperature in hot and cold environments
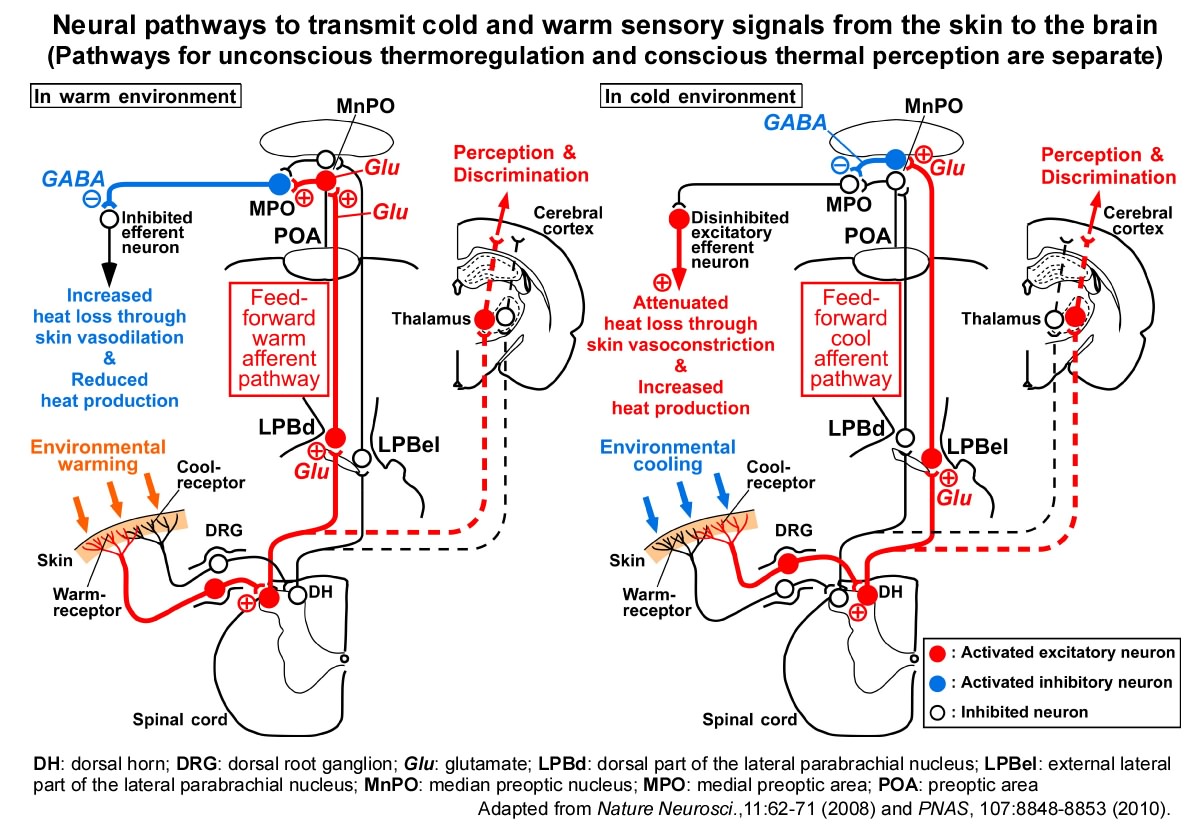
Adaptive heat production (thermogenesis) in a cold environment, such as shivering, is triggered by cold sensation delivered from thermoreceptors in the skin to the thermoregulatory brain center, preoptic area (POA) of the hypothalamus. However, the neural circuit mechanism by which such temperature sensory information is transmitted from the skin to the POA had long been a big question until we determined it in 2008.
We discovered that 1) thermosensory information from the skin is transmitted to the lateral parabrachial nucleus (LPB) of the brainstem through the spinal cord, that 2) this information is further transmitted from the LPB to the POA, and that 3) cold and warm sensory pathways are mediated by separate populations of neurons in the LPB. Rats whose spinal-LPB-POA neural pathways are interrupted cannot promptly produce heat in a cold environment, dissipate body heat in a hot environment, or choose comfort thermal environment, indicating that these thermosensory neural pathways play a critical role in autonomic and behavioral defense of body temperature from ambient cold and heat.
We also ablated another thermosensory neural pathway that is well-known to transmit thermosensory information from the skin to the cerebral cortex to "feel" skin temperature (spinothalamocortical pathway). However, it did not affect autonomic responses for body temperature regulation. These findings indicate that in a cold or hot environment, the conscious feeling (perception) of the ambient temperature and the homeostatic responses to defend body temperature are simultaneously driven by the different thermosensory neural pathways (Nakamura & Morrison, Nature Neurosci., 2008; J. Physiol., 2008; PNAS, 2010; Yahiro et al., Sci. Rep., 2017; J. Neurosci., 2023).
2) "Fever Switch" in the brain
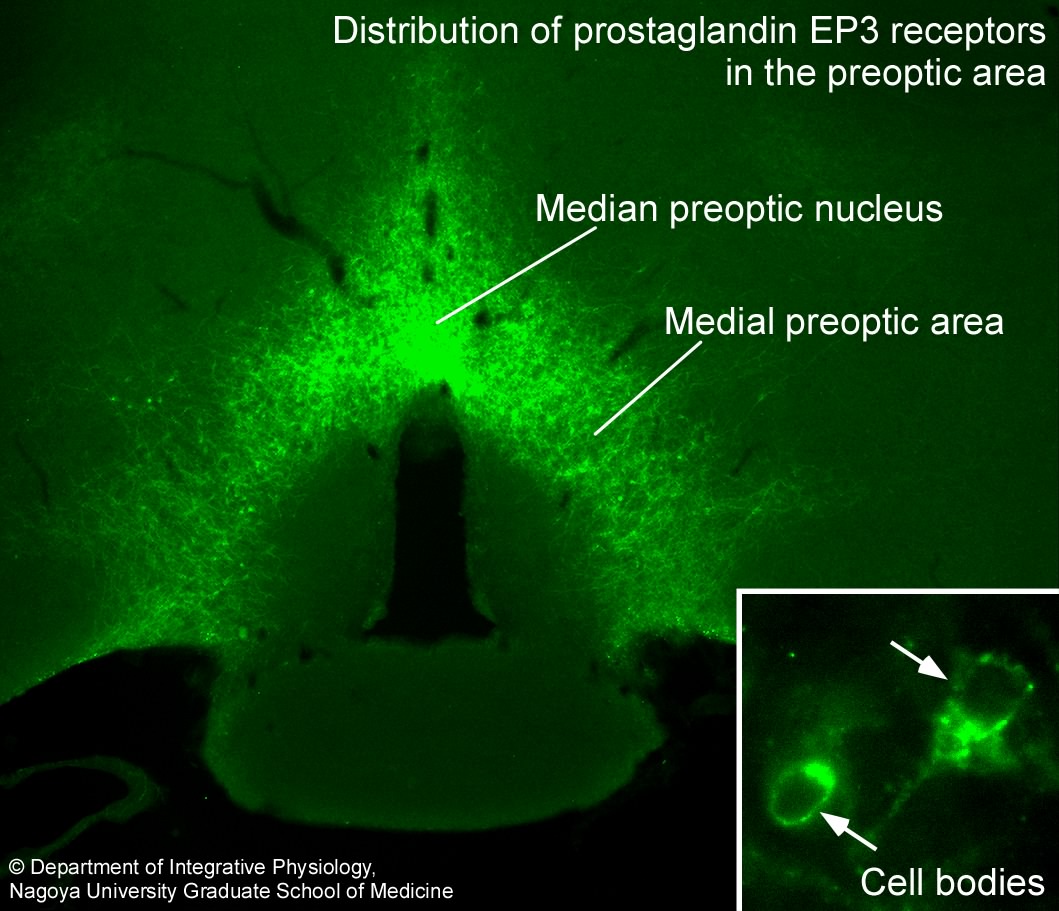 The POA is the febrile center as well as the center for body temperature regulation. Action of PGE2, a pyrogenic mediator produced following infection, in the POA triggers activation of the neural circuit for the development of fever. In 1998, we succeeded in visualizing the "fever switch"—prostaglandin EP3 receptors expressed in POA neurons, on which PGE2 acts to trigger fever (Nakamura et al., Neurosci. Lett., 1999; J. Comp. Neurol., 2000).
The POA is the febrile center as well as the center for body temperature regulation. Action of PGE2, a pyrogenic mediator produced following infection, in the POA triggers activation of the neural circuit for the development of fever. In 1998, we succeeded in visualizing the "fever switch"—prostaglandin EP3 receptors expressed in POA neurons, on which PGE2 acts to trigger fever (Nakamura et al., Neurosci. Lett., 1999; J. Comp. Neurol., 2000).
We found that these EP3 receptor-expressing POA neurons send the febrile command signals to at least two brain regions: 1) dorsomedial hypothalamus (DMH) and 2) rostral medullary raphe region (rMR), both of which are involved in stimulation of sympathetic functions. Blockade of either region eliminated thermogenic responses to develop fever. Therefore, these regions mediate fever signaling from the POA to the sympathetic nervous system, whose activation leads to enhanced heat production in brown adipose tissue (BAT) and reduced heat-loss from the skin surface (Nakamura et al., J. Neurosci., 2002; Eur. J. Neurosci., 2005; Neuroscience, 2009).
3) Discovery of sympathetic premotor neurons for body temperature regulation and fever
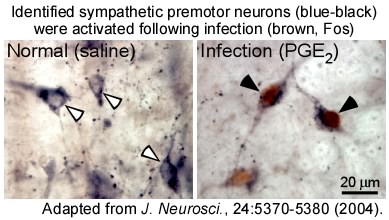 Our investigation of the role of the rMR in the regulation of the sympathetic nervous system led to the discovery of a novel population of sympathetic premotor neurons in this region that mediate the thermoregulatory and febrile signaling from the POA. These neurons are glutamatergic neurons expressing the vesicular glutamate transporter 3 (VGLUT3) and directly innervate sympathetic output (preganglionic) neurons in the spinal cord to command heat production in BAT and constriction of skin blood vessels. Importantly, when febrile and cold-defense responses are developed, these premotor neurons in the rMR are activated to drive these thermoregulatory sympathetic responses. We reported that these thermoregulatory sympathetic premotor neurons are different from sympathetic premotor neurons known to function for blood pressure control (Nakamura et al., J. Neurosci., 2004; NeuroReport, 2004; Neurosci. Res., 2005).
Our investigation of the role of the rMR in the regulation of the sympathetic nervous system led to the discovery of a novel population of sympathetic premotor neurons in this region that mediate the thermoregulatory and febrile signaling from the POA. These neurons are glutamatergic neurons expressing the vesicular glutamate transporter 3 (VGLUT3) and directly innervate sympathetic output (preganglionic) neurons in the spinal cord to command heat production in BAT and constriction of skin blood vessels. Importantly, when febrile and cold-defense responses are developed, these premotor neurons in the rMR are activated to drive these thermoregulatory sympathetic responses. We reported that these thermoregulatory sympathetic premotor neurons are different from sympathetic premotor neurons known to function for blood pressure control (Nakamura et al., J. Neurosci., 2004; NeuroReport, 2004; Neurosci. Res., 2005).
4) Central efferent neural pathways for body temperature regulation and fever development
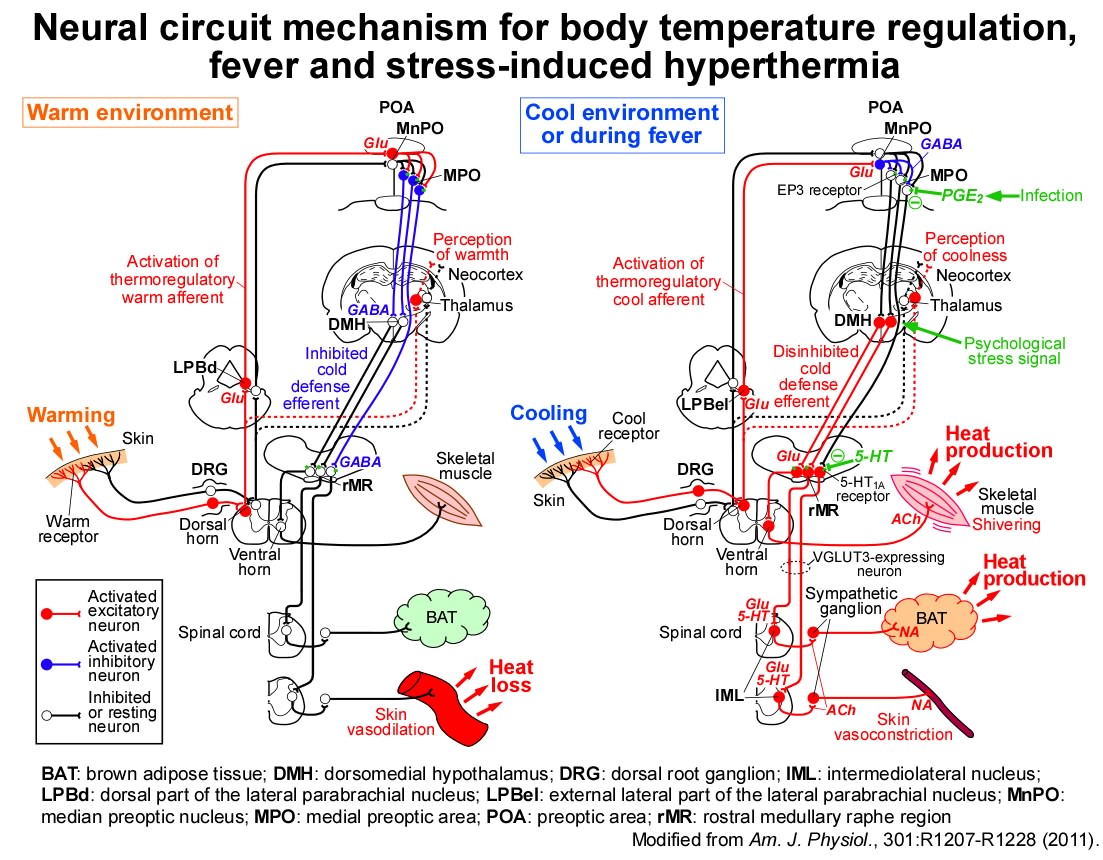
Then, how does the POA send the thermoregulatory command signals to the sympathetic premotor neurons in the rMR? We found that inhibition of neurons in the DMH and rMR, which mediate fever signaling from the POA, eliminated metabolic heat production in BAT as well as shivering in skeletal muscles in response to skin cooling (Nakamura & Morrison, Am. J. Physiol., 2007; J. Physiol., 2011). Our findings indicate that cold-defensive thermogenic signaling and fever-developing command signaling are both mediated by the same efferent pathways from the POA to peripheral thermogenic organs, and therefore, suggest that fever is a host-defense response to reduce pathogens' growth by temporarily over-activating the cold-defensive efferent pathways.
Recently, we have focused on a group of POA neurons expressing prostaglandin EP3 receptors (EP3 neurons) and have successfully regulated their neuronal activity by using molecular biological methodology as well as electrophysiological and neuroanatomical techniques developed to date. As a result, we elucidated that EP3 neurons serve as a "master controller" of body temperature regulation and fever, by constantly releasing the inhibitory neurotransmitter GABA and modulating the sympathetic pathways via the DMH and rMR (Nakamura et al. Science Adv., 2022).
5) Central circuit mechanism driving hunger responses to survive starvation
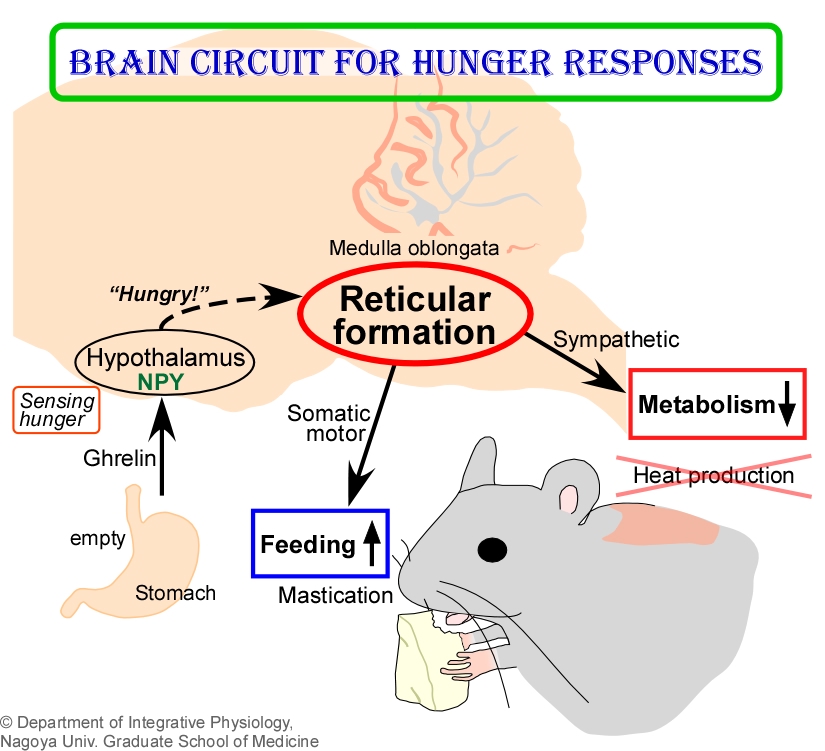
By combining techniques of in vivo physiology, neuroanatomy, and molecular biology, we recently discovered a novel group of neurons important for mammals to survive starvation. This neuronal group is distributed in the retimular formation of the medulla oblongata and activated by receiving "hunger signals" from the hypothalamus that are generated when animals are hungry. Curiously, we found that these activated reticular neurons inhibit metabolic heat production in BAT to save energy as well as stimulate mastication and feeding to facilitate energy intake. Under starved conditions, the same group of reticular neurons was found to take control of BAT sympathetic premotor neurons in the rMR for metabolic inhibition and of masticatory motoneurons for promotion of food intake. Presumably, this circuit mechanism has been essential for mammals to survive the long battle with starvation during evolution (Y. Nakamura et al., Cell Metab., 2017; Pflügers Arch., 2018).
6) Psychological effects on body temperature regulation and other autonomic functions
Anyone feels faster and stronger heart beating when being nervous or receiving psychological stress. This is a physiological response caused by a sympathetic drive to the heart, which is stimulated by emotion or psychological stress. However, the central neural circuit mechanism by which emotion and stress stimulate the brain system controlling such homeostatic autonomic functions remains unknown.
As expressed in the sayings "Your mind controls your body" and "Most illness is psychosomatic", we empirically know that strong psychological stress and emotional pressures have a strong impact on various vital systems in the body, such as the autonomic nervous system, endocrine system and immune system, leading to various diseases and at worst, a crisis of life. Therefore, research to understand how stress and emotion affect the homeostatic systems is important in medicine.
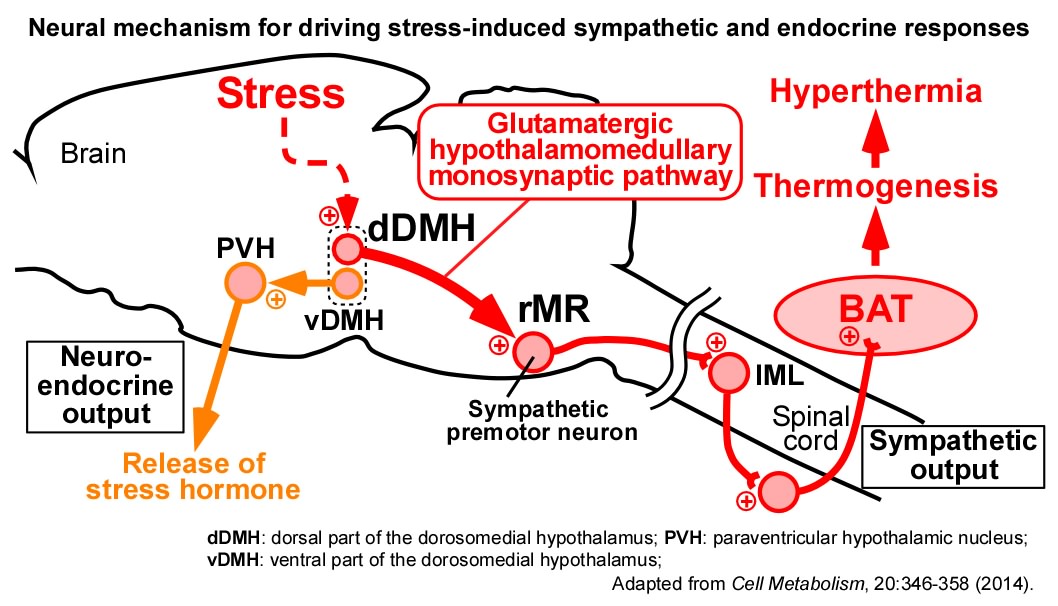
We have found that psychological stress activates the direct excitatory neurotransmission from the DMH to sympathetic premotor neurons in the rMR to stimulate BAT thermogenesis, resulting in the development of stress-induced hyperthermia (or psychogenic fever). We also found that stress-activated neurons in the DMH act as a hub in the stress circuit to drive the sympathetic stress responses (e.g., hyperthermia and tachycardia) and the neuroendocrine stress responses (e.g., release of stress hormones) (Lkhagvasuren et al., Eur. J. Neurosci., 2011; Neuroscience, 2014; Kataoka et al., Cell Metab., 2014).
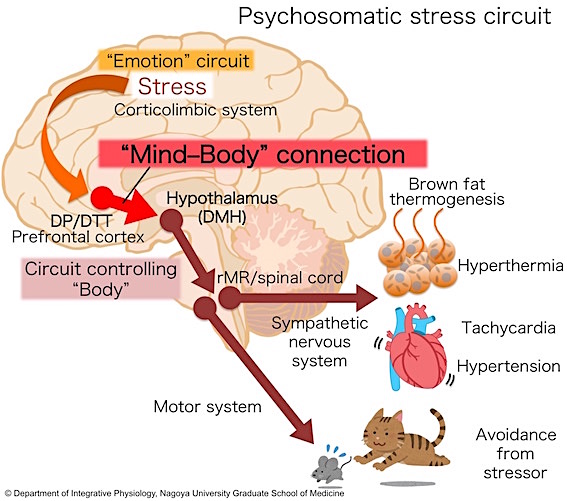
Recently, we further discovered a key circuit mechanism by which stress signals are transmitted from the emotion circuits in the cerebral cortex and limbic system to the DMH, which controls the body, to drive a variety of sympathetic stress responses as well as avoidance behavior from stressors. This discovery of the "mind–body connection" in the brain is an important step to understand the mechanism of aberrant physiological responses in stress-related disorders including psychogenic fever, panic disorder and post-traumatic stress disorder (PTSD) (Kataoka et al., Science, 2020).
Copyright (c) 2009-2025 Department of Integrative Physiology, Nagoya University Graduate School of Medicine
 Human body temperature is maintained at approximately 37°C. The body core temperature elevated or lowered by a few degrees Celcius from the optimal range causes many problems in body functions and can lead to death. So, the body has the systems that prevent elevation of body temperature in hot environments by facilitating sweating and that keep the body warm in cold environments by producing heat through shivering. The systems controlling these homeostatic responses are located in the brain and function independently of our will and consciousness.
Human body temperature is maintained at approximately 37°C. The body core temperature elevated or lowered by a few degrees Celcius from the optimal range causes many problems in body functions and can lead to death. So, the body has the systems that prevent elevation of body temperature in hot environments by facilitating sweating and that keep the body warm in cold environments by producing heat through shivering. The systems controlling these homeostatic responses are located in the brain and function independently of our will and consciousness. Fever is a common symptom developed following infection with viruses and bacteria (imagine a cold or flu). This is a host-defense response that reduces the growth of pathogens in the body. When infection occurs, the immune system is activated and communicates to the brain, resulting in the production of the pyrogenic mediator, prostaglandin E
Fever is a common symptom developed following infection with viruses and bacteria (imagine a cold or flu). This is a host-defense response that reduces the growth of pathogens in the body. When infection occurs, the immune system is activated and communicates to the brain, resulting in the production of the pyrogenic mediator, prostaglandin E

 Our investigation of the role of the rMR in the regulation of the sympathetic nervous system led to the discovery of a novel population of sympathetic premotor neurons in this region that mediate the thermoregulatory and febrile signaling from the POA. These neurons are glutamatergic neurons expressing the vesicular glutamate transporter 3 (VGLUT3) and directly innervate sympathetic output (preganglionic) neurons in the spinal cord to command heat production in BAT and constriction of skin blood vessels. Importantly, when febrile and cold-defense responses are developed, these premotor neurons in the rMR are activated to drive these thermoregulatory sympathetic responses. We reported that these thermoregulatory sympathetic premotor neurons are different from sympathetic premotor neurons known to function for blood pressure control (Nakamura et al.,
Our investigation of the role of the rMR in the regulation of the sympathetic nervous system led to the discovery of a novel population of sympathetic premotor neurons in this region that mediate the thermoregulatory and febrile signaling from the POA. These neurons are glutamatergic neurons expressing the vesicular glutamate transporter 3 (VGLUT3) and directly innervate sympathetic output (preganglionic) neurons in the spinal cord to command heat production in BAT and constriction of skin blood vessels. Importantly, when febrile and cold-defense responses are developed, these premotor neurons in the rMR are activated to drive these thermoregulatory sympathetic responses. We reported that these thermoregulatory sympathetic premotor neurons are different from sympathetic premotor neurons known to function for blood pressure control (Nakamura et al., 


